Potency Assessment of DNA & RNA Drugs
Creative Proteomics stands as a leader in offering comprehensive services for assessing the potency of DNA & RNA drugs. With years of industry experience, our company possesses a profound understanding of the distinct challenges and prerequisites associated with evaluating the potency of DNA & RNA drugs.
Introduction of DNA & RNA Drug Potency Assessment
Potency assessment of DNA & RNA drugs involves the evaluation of their biological activity and the quantification of their capability to elicit the desired therapeutic response. This process necessitates the use of specialized assays designed for precise potency measurement. DNA & RNA drugs can modulate gene expression by targeting specific RNA or DNA molecules, employing various mechanisms such as antisense oligonucleotides, small interfering RNAs (siRNAs), and messenger RNA (mRNA) therapeutics. Potency assessment assumes a critical role in determining the biological activity and therapeutic potential of these drugs.
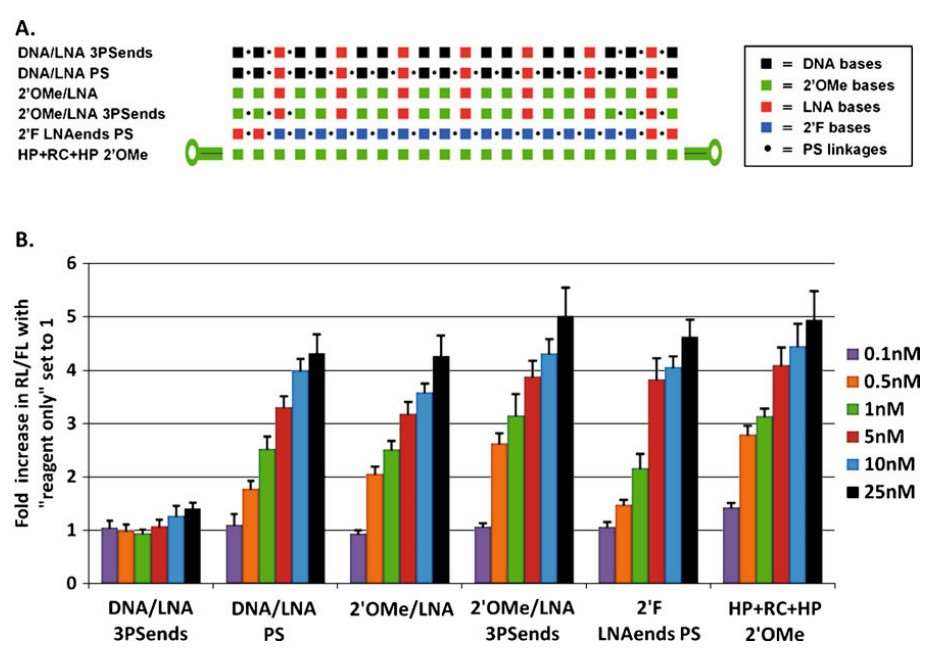 Fig. 1. A direct comparison of the potency of miR16 AMOs. (Lennox K. A., et al., 2010)
Fig. 1. A direct comparison of the potency of miR16 AMOs. (Lennox K. A., et al., 2010)
Our DNA & RNA Drug Potency Assessment Services
Creative Proteomics employs a diverse array of approaches in the assessment of DNA & RNA drug potency. We provide a spectrum of cutting-edge techniques and assays meticulously tailored for DNA & RNA drug potency evaluation. Our bioanalytical testing encompasses the quantification of DNA & RNA drugs and their metabolites across various biological matrices, employing advanced analytical methods like liquid chromatography and mass spectrometry. In vitro assays assess the drug's binding affinity to its target, cellular uptake, and its impact on gene expression. In vivo assays, frequently conducted in animal models, evaluate pharmacokinetics, tissue distribution, and the therapeutic effects of the drug. Here are some of the primary assays we offer.
Cell-Based Assays
Creative Proteomics employs an array of cell-based assay models, encompassing cell migration assays, cell signaling assays, cell proliferation/inhibition assays, and binding/competitive assays. These assays yield invaluable insights into the drug's mechanism of action and its capacity to attain the desired therapeutic outcome.
Ligand and Receptor Binding Assays
Creative Proteomics utilizes techniques such as Enzyme-Linked Immuno-Sorbent Assays (ELISA) and Surface Plasmon Resonance (SPR) to gauge the drug's affinity to its target. These assays offer a direct assessment of the drug's binding characteristics and contribute to the characterization of its interaction with the intended target.
Physicochemical Assays
In addition to cell-based and binding assays, Creative Proteomics employs physicochemical assessments to gauge the effectiveness of DNA & RNA drugs. These assessments delve into the drug's structural attributes, post-translational modifications, and other physicochemical traits that could influence its biological activity. Methods such as molecular weight determination, electrophoretic profiling, chromatographic analysis, and spectroscopic techniques are utilized in this evaluation.
DNA & RNA Drug Potency Assessment Application Fields
Assessing potency holds pivotal significance in DNA & RNA drug development and regulatory approval. It furnishes vital insights into the drug's biological functionality, efficacy, and safety profile. The application of potency assessment spans across several stages in the drug development process:
- Candidate Selection.
- Product Characterization.
- Biopharmaceutical Stability Testing.
- Biologic Product Release Testing.
Creative Proteomics delivers comprehensive services for evaluating the potency of DNA & RNA drugs, employing advanced techniques and assays. Leveraging our expertise, customized approaches, and adherence to regulatory standards, we offer precise and dependable results to facilitate the development and regulatory approval of DNA & RNA drugs. Feel free to contact us for discussions regarding your DNA & RNA drug potency assessment requirements.
Reference
- Lennox K. A.; Behlke M. A. (2010). A direct comparison of anti-microRNA oligonucleotide potency. Pharmaceutical research. 27(9):1788-1799.
For research use only, not intended for any clinical use.

 Fig. 1. A direct comparison of the potency of miR16 AMOs. (Lennox K. A., et al., 2010)
Fig. 1. A direct comparison of the potency of miR16 AMOs. (Lennox K. A., et al., 2010)