Molecular Weight Confirmation of DNA & RNA Drugs
Introduction of DNA & RNA Drug Molecular Weight Confirmation
The molecular weight of DNA & RNA refers to the sum of the atomic masses of all constituent atoms within a specific DNA & RNA sequence. These sequences are typically composed of short stretches of nucleotides, including adenine (A), cytosine (C), guanine (G), and thymine (T) in DNA, as well as uracil (U) in RNA. Molecular weight is quantified in Daltons (Da) or kilodaltons (kDa) and stands as a pivotal parameter in characterizing DNA & RNA drugs, elucidating their physical and chemical attributes. Commonly employed methods for determining the molecular weight of DNA & RNA encompass ESI-MS, LC-MS, and MALDI-TOF.
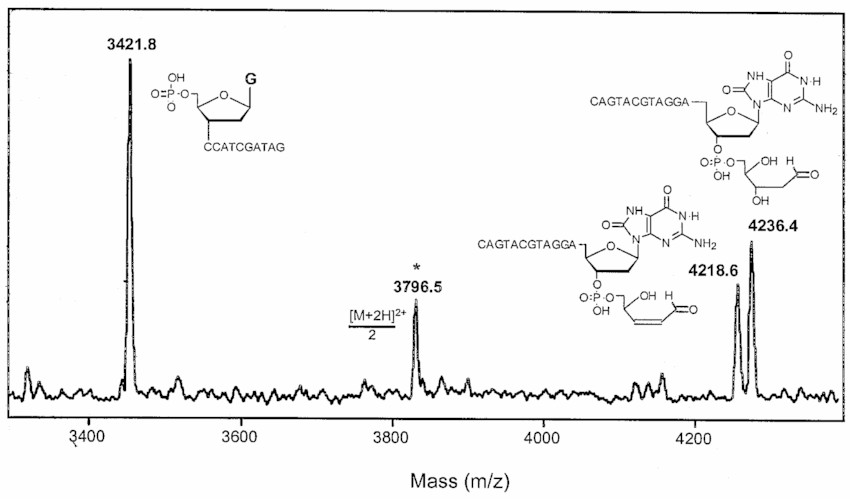 Fig. 1. MALDI-TOF mass spectrum of products resulting from the incubation of oligonucleotide with endonuclease III. (Bourdat A., et al., 1999)
Fig. 1. MALDI-TOF mass spectrum of products resulting from the incubation of oligonucleotide with endonuclease III. (Bourdat A., et al., 1999)
Our DNA & RNA Drug Molecular Weight Confirmation Services
Creative Proteomics offers a comprehensive suite of services for DNA & RNA molecular weight determination. With our cutting-edge facilities and proficient team, we guarantee the delivery of precise and reliable results. We harness advanced technologies, such as mass spectrometry, to ascertain the molecular weight of DNA & RNA sequences with exceptional accuracy. Some of the commonly utilized methods include:
MALDI-TOF Analysis
We provide MALDI-TOF analysis, a technique tailored for the detection of large DNA and RNA molecules. This procedure entails the application of a matrix to facilitate ionization, succeeded by time-of-flight mass analysis. A laser is employed to irradiate the matrix, resulting in the ionization and release of DNA & RNA molecules into the gaseous phase. The ions are then accelerated in an electric field and separated according to their time of flight to the detector. The time taken for an ion to reach the detector is proportional to its mass-to-charge ratio (m/z), enabling the determination of molecular weight.
LC-MS Analysis
We offer LC-MS services to analyze complex mixtures of DNA and RNA drugs because it allows for the separation of different components prior to mass analysis, resulting in better resolution and identification of impurities or truncated sequences. The analytical process consists of two main steps: liquid chromatography separation and mass spectrometry analysis. DNA and RNA drugs are separated using stationary and mobile phases based on their chemical properties. After separation, the eluted DNA and RNA drugs enter the mass spectrometer for molecular weight determination.
ESI-MS Analysis
We offer ESI-MS analysis services that gently ionize large biomolecules without creating visible debris. The synthesized DNA & RNA is typically dissolved in a volatile solvent. The solution containing the DNA & RNA is infused through a capillary needle held at high voltage. The high electric field at the needle tip causes the DNA & RNA molecules to ionize, forming gas-phase ions. The ions are then introduced into the mass spectrometer, where they are separated based on their mass-to-charge ratio (m/z). The detector records the intensity of ions at different m/z values, creating a mass spectrum. The mass spectrum provides information about the charge state and molecular weight of the DNA & RNA. The molecular weight is calculated from the m/z value, considering the charge state and any adduct ions present.
DNA & RNA Drug Molecular Weight Confirmation Application Fields
The molecular weight confirmation of DNA & RNA drugs finds application in various fields, spanning research, diagnostics, and industrial processes.
- Quality Control in Oligonucleotide Synthesis
- Structural Insights
- Biological Function
- Drug Development
- Pharmacokinetics and Pharmacodynamics
- Nanotechnology
Partner with Creative Proteomics today, and unlock the full potential of your DNA & RNA drugs research. Let our expertise drive your success. Contact us now to discuss your specific requirements or to request a quote for our DNA & RNA molecular weight confirmation services.
Reference
- Bourdat A.; et al. (1999). Synthesis and enzymatic processing of oligodeoxynucleotides containing tandem base damage. Nucleic Acids Res. 27(4):101-1024.
For research use only, not intended for any clinical use.

 Fig. 1. MALDI-TOF mass spectrum of products resulting from the incubation of oligonucleotide with endonuclease III. (Bourdat A., et al., 1999)
Fig. 1. MALDI-TOF mass spectrum of products resulting from the incubation of oligonucleotide with endonuclease III. (Bourdat A., et al., 1999)