Moisture Content Determination Service of DNA & RNA Drugs
Creative Proteomics is committed to excellence in providing comprehensive and cutting-edge solutions for the pharmaceutical and biotechnology industries. Our DNA & RNA drug moisture content determination service is designed to meet the stringent quality control demands of DNA & RNA drug development and manufacturing.
Introduction of DNA & RNA Drug Moisture Content Determination
DNA & RNA drug moisture content determination is a quality control process aimed at determining the amount of moisture present in DNA & RNA drug samples. Moisture content can directly impact the stability and efficacy of these drugs. High moisture levels can lead to degradation, reduced shelf life, and altered pharmacological properties, compromising their therapeutic effects. Thus, precise and accurate measurement of moisture content is essential to ensure the safety and efficacy of DNA & RNA drugs.
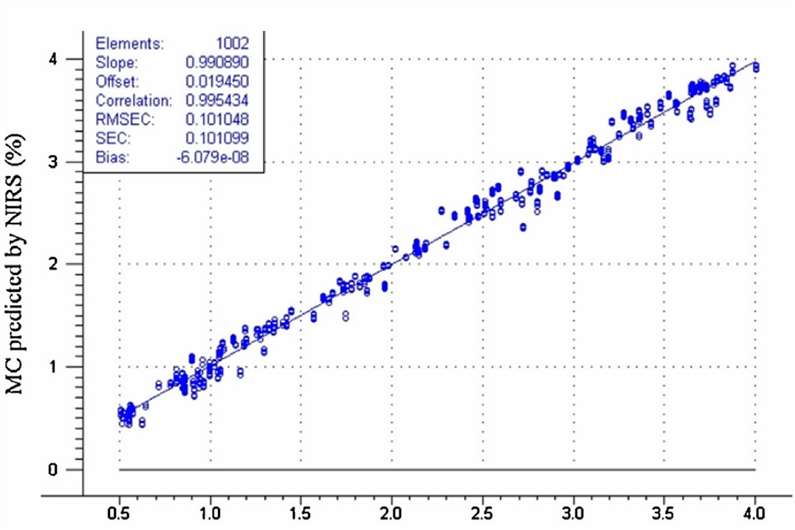 Fig. 1. Moisture content predicted by the NIRS method versus KF titration. (Clavaud M., et al., 2016)
Fig. 1. Moisture content predicted by the NIRS method versus KF titration. (Clavaud M., et al., 2016)
Our Moisture Content Determination Services
Creative Proteomics provides accurate moisture content determination services to ensure the stability, activity and performance of DNA & RNA drugs, thus ensuring their reliability and effectiveness in various applications. Below are a few of our commonly used moisture content determination methods.
Karl Fischer Titration
We offer Karl Fischer titration determination services, which is a widely used technique for moisture content determination. The method is highly accurate and provides precise measurement of low moisture content.
Thermogravimetric Analysis (TGA)
We offer services for TGA, which is a technique used to determine the thermal properties of a sample. In TGA, the sample is heated at a certain rate of warming while its weight loss is measured. The evaporation of water results in a reduction in the sample's mass, allowing the moisture content of the sample to be calculated.
Infrared Spectroscopy (IR)
The IR spectroscopy service we offer can be used to determine the moisture content in an DNA & RNA sample. The presence of moisture causes specific infrared absorption peaks, and by analyzing the infrared spectroscopy profile the moisture content in the sample can be determined.
Gas Chromatography (GC)
The GC we offer can also be used to determine the moisture content in DNA & RNA drugs. In GC, the sample is evaporated and enters the column with a carrier gas, and the moisture content is calculated by measuring the retention time of the moisture on the column.
Process of Moisture Content Determination
The process of DNA & RNA drugs moisture content determination involves several steps.
Sample Collection and Preparation
Our experts collect representative samples from your DNA & RNA drug batches and ensure proper sample preparation for Determination.
Method Selection
Based on the sample's properties and the expected moisture content range, we choose the most suitable analytical method to deliver accurate results.
Precise Analysis
Our advanced instrumentation and rigorous determination protocols guarantee precise measurement of moisture content.
Data Interpretation and Reporting
We analyze the obtained data and prepare detailed reports, providing insights into your DNA & RNA drugs' moisture content and its potential impact on stability and efficacy.
Moisture Content Determination Application Fields
- Stability Studies.
- Batch-to-Batch Consistency.
- Drug Development and Formulation.
- Dose Optimization.
- Drug Repurposing.
Creative Proteomics is committed to providing scientific excellence, state-of-the-art technology, and personalized service to ensure that your DNA & RNA drugs meet the highest quality standards. With our support, you can confidently move forward in developing and manufacturing innovative DNA & RNA drugs that have the potential to transform the landscape of modern medicine. Contact us today to learn more about our moisture content determination service and how we can contribute to your success in the pharmaceutical industry.
Reference
- Clavaud M.; et al. (2016). Moisture content determination in an antibody-drug conjugate freeze-dried medicine by near-infrared spectroscopy: A case study for release testing. J Pharm Biomed Anal. 131:380-390.
For research use only, not intended for any clinical use.

 Fig. 1. Moisture content predicted by the NIRS method versus KF titration. (Clavaud M., et al., 2016)
Fig. 1. Moisture content predicted by the NIRS method versus KF titration. (Clavaud M., et al., 2016)