Impurity Profiling of DNA & RNA Drugs
Introduction of DNA & RNA Drug Impurity Profiling
DNA & RNA drug impurity profiling is a multifaceted process encompassing the intricate tasks of identifying, characterizing, and quantifying impurities within the DNA & RNA drug substances or drug products. These impurities can spring forth from a plethora of sources, ranging from the starting materials to the obstreperous reagents, and even the intermediates and manufacturing processes themselves.
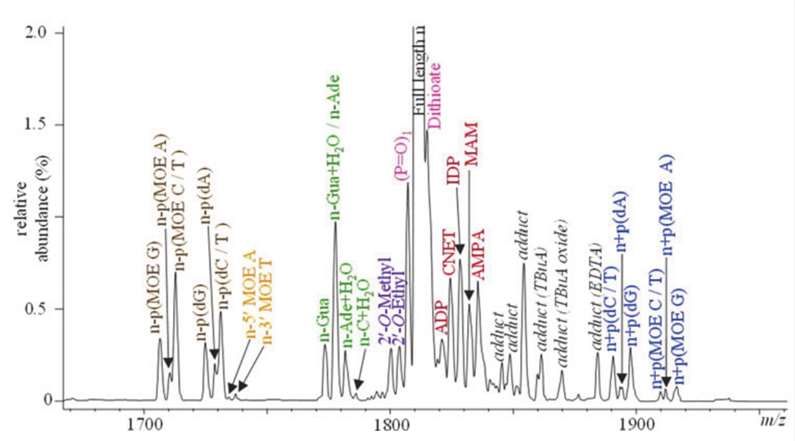 Fig. 1. Identification of co-eluting impurities in mass spectrum of IP-LCMS. (Rentel C, et al., 2022)
Fig. 1. Identification of co-eluting impurities in mass spectrum of IP-LCMS. (Rentel C, et al., 2022)
Our DNA & RNA Drug Impurity Profiling Services
Creative Proteomics offers customised DNA and RNA drug impurity analysis services with state-of-the-art instrumentation and an experienced team. We identify and quantify impurities using liquid chromatography (LC), mass spectrometry (MS) and capillary electrophoresis (CE). Our services include, but are not limited to, the following:
Method development
We design specific analytical methods based on the examination of DNA and RNA drugs. to ensure accurate and reliable results.
Impurity identification
We provide services to identify impurities in DNA and RNA drug products. Using advanced analytical techniques such as LC-MS, we can determine the exact quality and data of the impurities. This information is then cross-referenced to a reference to characterize the impurity and gain insight into its chemical properties.
Quantification
Creative Proteomics employs validated methods to quantify impurities in DNA & RNA drug samples. These methods typically involve the use of calibration standards and external/internal standards for accurate and reliable quantification. The quantitative results provide valuable information about the level of impurities and help establish acceptable limits for impurities in pharmaceutical products.
Process of DNA & RNA Drug Impurity Profiling
DNA & RNA drug impurity profiling involves a systematic process to ensure a comprehensive analysis of impurities. The process consists of the following main steps.
Sample Preparation
DNA & RNA drug samples are carefully prepared to extract impurities and remove any interfering substances. Sample preparation techniques may involve solid-phase extraction, precipitation, or enzymatic digestion.
Analytical Method Selection
Based on the characteristics of the DNA & RNA drug, an appropriate analytical method is selected, considering factors such as impurity type, concentration range, and regulatory guidelines.
Analysis and Detection
The prepared samples are subjected to analytical techniques such as LC, MS, or CE to separate and detect impurities. Mass spectrometry provides valuable structural information for impurity identification.
Impurity Characterization
Identified impurities are characterized by comparing their properties to reference standards or using spectral databases. Characterization helps in understanding the potential risks and origins of impurities.
Quantification
Impurity levels are quantified using suitable calibration standards and validated methods. Quantification allows for a comprehensive assessment of impurity content and compliance with regulatory limits.
DNA & RNA Drug Impurity Profiling Application Fields
Our DNA & RNA drug impurity profiling finds application at various stages of the drug development process, including research and development, manufacturing, and quality control.
- Method Development and Optimization.
- Process Validation.
- Regulatory Compliance.
- Post-Marketing Surveillance.
Creative Proteomics offers a range of impurity profiling services, employing advanced techniques to identify, quantify, and characterize impurities. As the field of DNA & RNA therapeutics continues to grow, the importance of comprehensive and accurate impurity profiling will remain paramount in the pursuit of safe and effective drug products. If you are interested in our services, please contact us for more detailed information.
Reference
- Rentel C, Gaus H, Bradley K, et al. (2022). Assay, Purity, and Impurity Profile of Phosphorothioate Oligonucleotide Therapeutics by Ion Pair-HPLC-MS. Nucleic Acid Ther. 32(3):206-220.
For research use only, not intended for any clinical use.

 Fig. 1. Identification of co-eluting impurities in mass spectrum of IP-LCMS. (Rentel C, et al., 2022)
Fig. 1. Identification of co-eluting impurities in mass spectrum of IP-LCMS. (Rentel C, et al., 2022)