Encapsulation Efficiency (VLP) Determination of DNA & RNA Drugs
At Creative Proteomics, we recognize the critical importance of effective drug delivery in ensuring the safety and efficacy of your DNA & RNA therapeutics. Our encapsulation efficiency determination services offer a comprehensive evaluation of drug formulations, guaranteeing optimal drug encapsulation and improved therapeutic outcomes.
Introduction of Encapsulation Efficiency (VLP) Determination
The determination of encapsulation efficiency serves as a vital technique for evaluating the effectiveness of encapsulating DNA & RNA drugs within virus-like particles (VLPs). VLPs are naturally forming nanoparticles derived from viruses, lacking genetic material and replication capability, rendering them both safe and highly efficient as carriers for DNA & RNA drugs. The primary objective of encapsulation efficiency determination is to ascertain the percentage of successfully encapsulated oligonucleotides within VLPs. This process provides valuable insights into the efficacy of the drug delivery system.
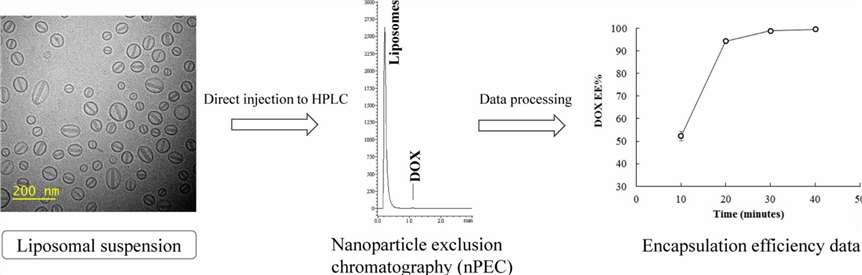 Fig. 1. Encapsulation efficiency determination of nanoparticles. (Yamamoto E., et al., 2018)
Fig. 1. Encapsulation efficiency determination of nanoparticles. (Yamamoto E., et al., 2018)
Our Encapsulation Efficiency (VLP) Determination Services
Creative Proteomics offers encapsulation efficiency (VLP) determination services to pharmaceutical companies and researchers involved in drug development. Our services encompass the following areas.
Characterization of VLPs
Comprehensive analysis of VLPs to determine their physical and chemical properties, including size, shape, and surface charge.
Encapsulation Efficiency (VLP) Determination
Our encapsulation efficiency (VLP) determination service is designed to evaluate the percentage of DNA & RNA drugs encapsulated within Viral-Like Particles. Our advanced analytical techniques, such as high-performance liquid chromatography (HPLC) and capillary electrophoresis (CE), allow us to obtain precise measurements of the encapsulated drug. Our experts will guide you through the entire process, from sample preparation to data interpretation, ensuring reliable results.
Stability Testing
Assessing the stability of VLP-encapsulated DNA & RNA drugs under different storage conditions, as well as during transportation and administration.
Release Kinetics
Evaluating the release kinetics of the encapsulated drugs from VLPs to understand their controlled release behavior.
Data Analysis and Interpretation
Our team of experts doesn't stop at providing raw data. We analyze and interpret the results in the context of your drug development goals. You will receive comprehensive insights and actionable recommendations to optimize your drug formulations for enhanced therapeutic impact.
Process of Encapsulation Efficiency (VLP) Determination
Creative Proteomics offers a VLP assay service that follows a rigorous step-by-step process to ensure reliability and accuracy.
Drug Loading
We first mix the DNA and RNA drugs with the VLP under controlled conditions to encapsulate the drugs in the VLP.
Purification
We remove the unencapsulated drug from the VLP formulation by purification, ensuring accurate quantification of the encapsulated drug.
Quantification
We quantify the encapsulated DNA and RNA drugs using various analytical techniques such as spectrophotometry, chromatography, or fluorescence detection.
Calculating Encapsulation Efficiency
We calculate the percentage of encapsulated drug by comparing the amount of encapsulated drug to the total amount of drug used in the process.
Data Analysis
Finally, we analyze the results to assess the efficiency of drug encapsulation and optimize formulation parameters for further drug development.
Encapsulation Efficiency (VLP) Determination Application Fields
The application of encapsulation efficiency (VLP) determination is wide-ranging and holds great promise in the field of molecular medicine.
- Targeted Delivery.
- Optimization of Formulation.
- Enhanced Drug Efficacy.
- Cancer Treatment.
Creative Proteomics is committed to providing the most reliable encapsulation efficiency (VLP) determination services available. Have questions or need further information about our encapsulation efficiency (VLP) determination services? Contact us today! We're here to support you at every stage of your drug development journey.
Reference
- Yamamoto E.; et al. (2018). A simple and rapid measurement method of encapsulation efficiency of doxorubicin loaded liposomes by direct injection of the liposomal suspension to liquid chromatography. Int J Pharm. 536(1):21-28.
For research use only, not intended for any clinical use.

 Fig. 1. Encapsulation efficiency determination of nanoparticles. (Yamamoto E., et al., 2018)
Fig. 1. Encapsulation efficiency determination of nanoparticles. (Yamamoto E., et al., 2018)