LNP Composition Analysis for DNA & RNA Drugs
Creative Proteomics provides extensive characterization services for therapeutic DNA and RNA molecules. Our specialized DNA and RNA drug LNP composition analysis services are designed to address the specific requirements of drug development and therapeutic research. Our expert analysis yields valuable insights into the formulation of lipid nanoparticle (LNP) delivery systems.
Introduction of DNA & RNA Drug LNP Composition
Lipid nanoparticles (LNPs) are minute particles constructed from lipids, tasked with encapsulating therapeutic DNA & RNA. LNPs function as delivery carriers, safeguarding the delicate DNA & RNA cargo during its journey through the body and facilitating its entry into targeted cells. The composition of LNPs is meticulously crafted, comprising an array of lipids, surfactants, and stabilizing agents to ensure stability, efficient encapsulation, and controlled release of the therapeutic payload.
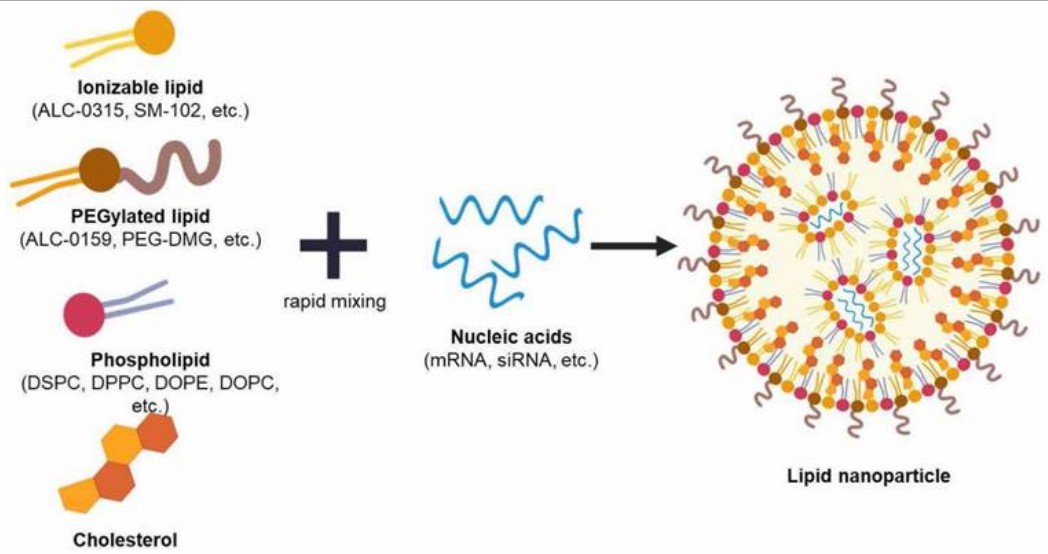 Fig. 1. Illustration of lipid nanoparticle and its compositions. (Jung HN, et al., 2022)
Fig. 1. Illustration of lipid nanoparticle and its compositions. (Jung HN, et al., 2022)
Our LNP Composition Analysis Services
Creative Proteomics extends its services for the analysis of LNP composition in DNA & RNA drug formulations. Leveraging a dedicated team of seasoned scientists and cutting-edge equipment, we offer a comprehensive array of services tailored to fulfill the distinctive requirements of our clients. Our LNP composition analysis services encompass the following domains.
Lipid Identification
We perform a comprehensive lipidomic analysis to identify and quantify the various lipids present in your LNP formulation.
Quantitative Analysis
Accurate quantification of lipids and DNA & RNA drugs within the LNPs is provided, giving insights into encapsulation efficiency and drug-loading capacity.
Particle Size and Morphology
In analyzing the size and shape of LNPs, crucial factors influence their circulation time and cellular uptake.
Stability Analysis
We evaluate the stability of LNPs under various conditions, providing insights into drug release kinetics during storage and administration.
Purity and Contaminant Assessment
We identify and quantify potential impurities or contaminants in the LNP formulation, ensuring product quality and safety.
Process of LNP Composition Analysis
The process of LNP composition analysis involves several key steps.
Sample Preparation
LNPs are isolated and purified from the drug formulation for analysis, ensuring accurate results.
Lipid Extraction
Lipids are extracted from the isolated LNPs using appropriate methods, preserving their integrity for further analysis.
Instrumental Analysis
Various advanced techniques, such as mass spectrometry, nuclear magnetic resonance (NMR), and chromatography, are employed for lipid and DNA & RNA identification and quantification.
Data Interpretation
Bioinformatics tools and software are utilized to interpret the obtained data and generate comprehensive reports.
LNP Composition Analysis Application Fields
The application of LNP composition analysis is wide-ranging and holds great promise in the field of molecular medicine.
- Enhancing Drug Efficacy.
- Tailored Drug Design.
- Drug delivery system optimization.
- Neurological Disorders.
Advantages of Our Services
Data Accuracy
Our team of skilled scientists and bioinformaticians ensures accurate data analysis and interpretation, delivering reliable results.
Confidentiality
We understand the importance of data security and ensure strict confidentiality in handling your valuable samples and information.
Timely Delivery
We adhere to strict timelines to provide you with results when you need them, ensuring minimal delays in your research progress.
Creative Proteomics is committed to empowering researchers and pharmaceutical companies in harnessing the potential of LNP-based drug delivery, ultimately translating scientific innovation into improved patient care. Contact us to discuss your LNP composition analysis needs.
Reference
- Jung HN; et al. (2022). Lipid nanoparticles for delivery of RNA therapeutics: Current status and the role of in vivo imaging. Theranostics. 12(17):7509-7531.
For research use only, not intended for any clinical use.

 Fig. 1. Illustration of lipid nanoparticle and its compositions. (Jung HN, et al., 2022)
Fig. 1. Illustration of lipid nanoparticle and its compositions. (Jung HN, et al., 2022)