Forced Degradation Studies of DNA & RNA Drugs
Introduction of DNA & RNA Drug Forced Degradation Studies
Stability-assessment methods, including forced degradation studies, play a pivotal role in scrutinizing degradation pathways, impurity profiles, and the overall stability of pharmaceuticals. Forced degradation studies of DNA & RNA drugs entail subjecting the drug to rigorous conditions, such as elevated temperature, exposure to light, varying pH levels, and oxidative stress, in order to expedite its degradation. This accelerated degradation offers valuable insights into the pathways of decomposition, identification of potential degradation byproducts, and evaluation of the drug's resilience under stress conditions
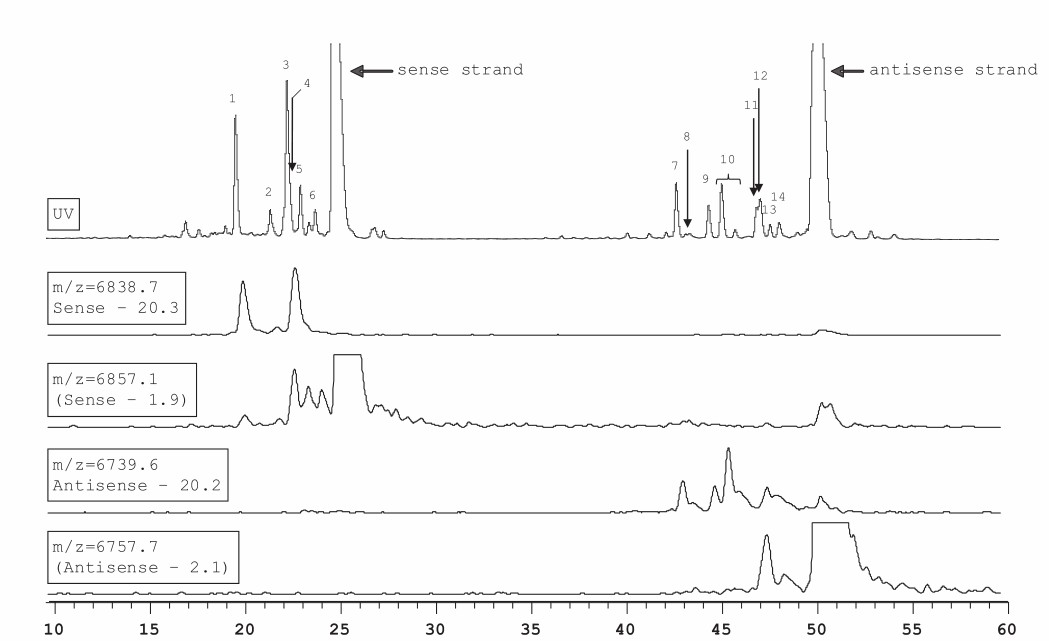 Fig. 1. Overlay of UV and extracted ion chromatograms for solid sample purged with nitrogen and stored at 60°C. (Rentel C, et al., 2022)
Fig. 1. Overlay of UV and extracted ion chromatograms for solid sample purged with nitrogen and stored at 60°C. (Rentel C, et al., 2022)
Our DNA & RNA Drug Forced Degradation Studies Services
Creative Proteomics specializes in delivering cutting-edge services for forced degradation studies of DNA & RNA drugs. Our proficiency and employment of advanced analytical techniques enable us to meticulously assess the stability and degradation characteristics of DNA & RNA-based drugs. Backed by a dedicated team of seasoned scientists and state-of-the-art equipment, we offer a comprehensive suite of services meticulously tailored to address the unique requirements of our clients. Our services encompass the following domains.
Method Development and Validation
Creative Proteomics excels in the development and validation of stability-indicating methods tailored for DNA & RNA drugs. Leveraging our extensive knowledge and experience, we engineer robust analytical procedures capable of effectively detecting alterations in the quality attributes of both drug substances and products throughout their storage lifecycle. Our method validation process guarantees the production of reliable and accurate data for regulatory submissions.
Forced Degradation Studies
Creative Proteomics employs a stringent approach in conducting forced degradation studies, exposing DNA & RNA drugs to a spectrum of stress conditions encompassing heat, light, humidity, oxidation, and acid/base hydrolysis. These stress conditions, meticulously aligned with regulatory guidelines, are thoughtfully crafted to induce controlled degradation and replicate potential degradation pathways that may arise during drug manufacturing, storage, and utilization.
Comprehensive Analytical Characterization
Within our state-of-the-art laboratory facilities, we conduct exhaustive analytical characterization of DNA & RNA drugs under forced degradation conditions. Employing advanced techniques such as ion-pairing reversed-phase high-performance liquid chromatography (IP-RP-HPLC), coupled with ultraviolet (UV) and mass spectrometry (MS) detection methodologies, we assess purity, impurity profiles, and degradation products of DNA & RNA drugs. This meticulous analysis enables us to acquire precise and comprehensive insights into structural modifications and degradation pathways.
Data Analysis and Reporting
Creative Proteomics ensures meticulous data analysis and comprehensive reporting of forced degradation studies. Our team of experts meticulously evaluates the obtained data, performs statistical analysis, and presents the findings clearly and concisely. Our reports include detailed information on the degradation products, quantification of impurities, and recommendations for optimizing drug stability.
Process of Forced Degradation Studies
DNA & RNA drug forced degradation studies typically follow a systematic process to ensure reliable and reproducible results.
Study Design
Defining the study's objectives, selecting appropriate stress conditions, and determining the analytical techniques required for analysis.
Sample Preparation
Preparing the drug samples according to established protocols, ensuring accurate concentrations and homogeneity.
Forced Degradation
Subjecting the drug samples to predetermined stress conditions, such as temperature, humidity, light, and pH, for a specified period.
Sample Analysis
Analyzing the stressed samples using suitable analytical methods to identify and quantify degradation products. Advanced techniques like HPLC, mass spectrometry, and NMR spectroscopy are commonly employed.
Data Interpretation
Evaluating the results obtained from the analysis, identifying degradation pathways, and characterizing the degradation products. Comparisons with reference samples or previous stability data may also be performed.
DNA & RNA Drug Forced Degradation Studies Application Fields
Forced degradation studies have wide-ranging applications in the development and evaluation of DNA & RNA drugs. There are some key applications.
- Prediction of Drug Stability.
- Identification of Degradation Products.
- Packaging and Storage Recommendations.
- Validation of Analytical Methods.
Creative Proteomics offers comprehensive services for DNA & RNA drug forced degradation, employing advanced analytical techniques, experienced scientists, and tailored solutions to meet the unique needs of our clients. By leveraging our expertise, we contribute to advancing pharmaceutical development and assuring high-quality DNA & RNA drugs in the market. If you are interested in our services, please contact us for more detailed information.
Reference
- Calvitt C, Levin D, Shepperd B, et al. (2010). Chemistry at the 2' position of constituent nucleotides controls degradation pathways of highly modified oligonucleotide molecules. Oligonucleotides. 20(5):239-251.
For research use only, not intended for any clinical use.

 Fig. 1. Overlay of UV and extracted ion chromatograms for solid sample purged with nitrogen and stored at 60°C. (Rentel C, et al., 2022)
Fig. 1. Overlay of UV and extracted ion chromatograms for solid sample purged with nitrogen and stored at 60°C. (Rentel C, et al., 2022)