Western blot was introduced by Towbin et al. in 1979, which is a commonly used method for protein analysis. It can be used for qualitative and semi-quantitative protein analysis. For the accomplishment of the western blot, there are three elements, separation of proteins by size, transferring proteins to a solid support, and marking proteins by primary and secondary antibodies for visualization.
The Principle of Western Blot
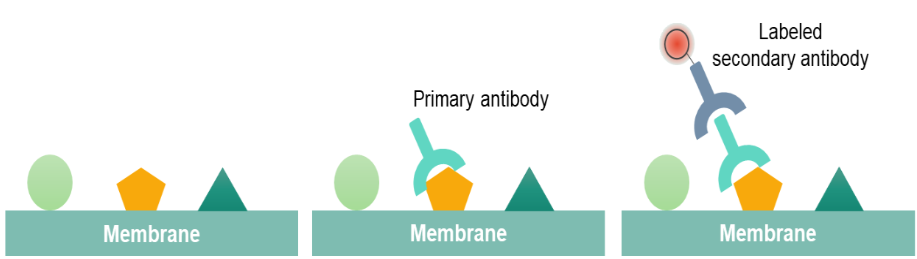
Western blot is performed by using polypropylene gel electrophoresis. SDS-PAGE allows protein samples to be separated and transferred to a solid support, such as nitrocellulose (NC) or polyvinylidene difluoride (PVDF) membrane. The solid support can absorb the protein and keep its biological activity unchanged. The transferred solid support membrane is called a blot and is treated with a protein solution to block the hydrophobic binding site on the membrane. The membrane is treated with the antibody (primary antibody) of the target proteins. Only the proteins to be studied can specifically bind to the primary antibody to form an antigen-antibody complex. After the primary antibody is washed and removed, only the position of the target protein binds to the primary antibody. The primary antibody-treated membranes are treated with a labeled secondary antibody after washing. After treatment, the labeled secondary antibody that binds to the primary antibody forms an antibody complex that can indicate the location of the primary antibody, both the location of the protein being studied.
The Procedure of Western Blot
There are six steps involved in western blot, including sample preparation, gel electrophoresis, proteins transfer, blocking, antibody incubation, and proteins detection and visualization.
1. Sample preparation.
Proteins can be extracted from different samples, such as tissues or cells. Since tissue samples display a higher degree of structure, the tissues are first broken down by the mechanical invention, such as homogenizer or sonication. Protease and phosphatase inhibitors are commonly used to prevent the digestion of the sample at cold temperatures. After protein extraction, it is important to detect the concentration of proteins, which permits the mass of proteins loaded into each well. And a spectrophotometer is often used for proteins concentration.
2. Gel electrophoresis.
The most commonly used gel is polyacrylamide gels (PAG) and buffers loaded with sodium dodecyl sulfate (SDS). Western blot uses two types of agarose gel: stacking gel that is used for concentrate all proteins in one band and separating gel that allows for separating proteins according to their molecular weight. Smaller proteins migrate faster in SDS-PAGE when a voltage is applied. PAGE can separate proteins ranging from 5 to 2,000 kDa according to the uniform pore size which is controlled by the Different concentration of PAG. Typically separating gels are made in 5%, 8%, 10%, 12% or 15%. When we choose the appropriate percentage of the separating gel, we should consider the size of the target proteins. The smaller the known weight of proteins is, the higher percentage of gels should be used.
3. Proteins transfer.
After separating proteins by gel electrophoresis, proteins are moved from within the gel onto a solid support membrane to make the proteins accessible to antibody detection. The main method for transferring proteins is called electroblotting, which uses an electric field oriented perpendicular to the surface of the gel, to pull proteins out of the gel and move into the membrane. It can be done semi-dry or wet conditions, while wet conditions are usually more reliable as it is less likely dry out the gel. As shown in the left figure, the membrane is placed between the gel surface and filter. The transfer sandwich is created as follows: a fiber pad (sponge), filter papers, the gel, a membrane, filter papers, a fiber pad (sponge).
4. Blocking.
Blocking is an important step in the western blot to prevent antibodies from binding to the membrane non-specifically. The most commonly used typical blockers are BSA and non-fat dry milk. When the membrane is placed in the dilute solution of proteins, the proteins attach to all places in the membrane where the target proteins have not attached. In this way, the "noise" in the final product of the western blot can be reduced and result in clearer results.
5. Antibody incubation.
After blocking, the primary antibody binds to target protein when the primary antibody is incubated with the membrane. The choice of a primary antibody depends on the antigen to be detected. Washing the membrane with the antibody-buffer solution is helpful for minimizing background and removes unbound antibodies. After rinsing the membrane, the membrane is exposed to the specific enzyme conjugated secondary antibody. When performing secondary antibody incubation, the labeled secondary antibody can bind to the primary antibody which has reacted with target proteins. Based on the species of the primary antibody, we can choose the appropriate secondary antibody.
6. Protein detection and visualization.
A substrate reacts with the enzyme that is bound to the secondary antibody to generate colored substance. It enables us to know the densitometry and location of the targets protein. And the size approximations are taken by comparing the proteins bands to the marker. There are several detection systems are available for protein visualization, such as colorimetric detection, chemiluminescent detection, radioactive detection, and fluorescent detection. The electrochemiluminescence (ECL) system is the most common detection method.
The western blot is commonly used for qualitative detection of proteins and post-translational modifications (e.g. phosphorylation). In addition, it also can be used in medical diagnostics, such as the HIV-test or BSE-test.
Reference:
- Mahmood T, Yang P C. Western blot: technique, theory, and trouble shooting. North American journal of medical sciences, 2012, 4(9): 429.