Methods in protein identification
Protein identification here refers to the accurate screening and identification of proteins using certain biochemical and physical methods. Traditional protein identification methods, including immunoblotting, comigration analysis of known or unknown proteins, and chemical sequencing of endopeptides, are complicated, time-consuming, and less accurate.
Protein identification methods have evolved from the image analysis technology, microsequencing, amino acid composition analysis, to mass spectrometry. The image analysis technology uses a spectrometer. The whole process involves detection of some spots, background subtraction, spot matching, and database construction. Image analysis and amino acid composition analysis need to be combined with other protein identification techniques to complete the whole process. Microsequencing technique has been updated rapidly, and the goal of automatic protein identification has been achieved. Nowadays, researchers are increasingly inclined to choose mass spectrometry-related technologies for rapid protein identification.
Different methods in protein identification by mass spectrometry
Mass spectrometer can compare the peptide fingerprint and the sequencing results of peptide fragments with the theoretical peptide mass fingerprinting (PMF) of the protein in the protein database to search for the best candidate protein. Methods for protein identification related to mass spectrometry include protein fingerprinting based on MALDI-TOF and peptide sequencing based on LC-MS/MS.
(1) The principle of the MALDI-TOF/MS
MALDI-TOF is a matrix-assisted laser desorption / ionization time-of-flight mass spectrometer. The MALDI-TOF-based protein fingerprinting method is used to digest a sample with a certain proteolytic enzyme (usually trypsin) and obtain an MS spectrum, which generates the mass of all peptides (or MH +). These qualities are used as fingerprints to search for proteins in the database and match the measured masses. The protein matched with the highest score is the candidate protein.
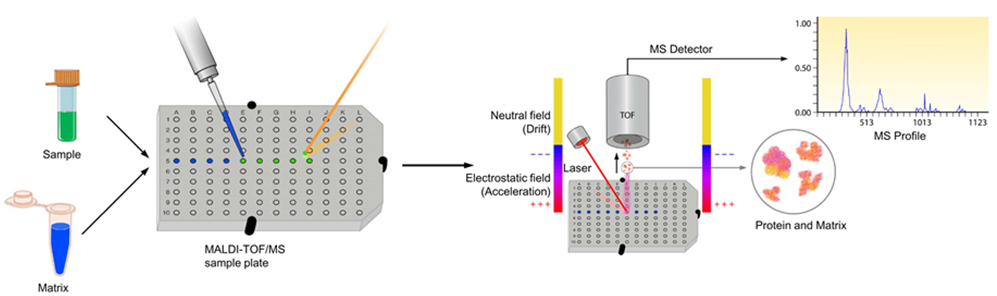
Figure 1. The process of MALDI-TOF mass spectrometry
(2) The advantage of the MALDI-TOF/MS
The advantage of MALDI-TOF/MS is high precision ion reflector and delayed ion extraction, which makes it fast to collect MALDI-TOF/MS data and analyze the result.
(3) The application of MALDI-TOF/MS
It is mainly used for the identification and classification of microorganisms in clinical medicine, food industry and animal and plant quarantine.
- LC-MS/MS
(1) The principle of the LC-MS/MS
The high-performance liquid chromatography-mass spectrometry system can be used to study the molecular structure and other information by analyzing the molecular weight and elemental composition of substances. During protein identification by LC-MS/MS peptide sequencing, the peptide mixture in proteolytic digestion (usually trypsin digestion) is separated by HPLC. Tandam mass spectrometer and HPLC are used to perform peptide fragmentation. MS/MS spectra are obtained for each fragmented peptide (usually there are thousands of MS/MS spectra for each sample). Each MS / MS spectrum (corresponding to a specific peptide sequence) is used to search the protein database for matching peptides to ultimately identify the protein. In particular, the combination of reversed-phase liquid chromatography and tandem mass spectrometry (tandem MS) can be detected at the level of dozens of picomole. If capillary chromatography is combined with tandem mass spectrometry, protein at low concentrations, a level less than femtomole and the atomic level, can be detected. At present, proteins are mostly identified by a combination of enzymatic hydrolysis, liquid chromatography separation, tandem mass spectrometry, and computer algorithms.
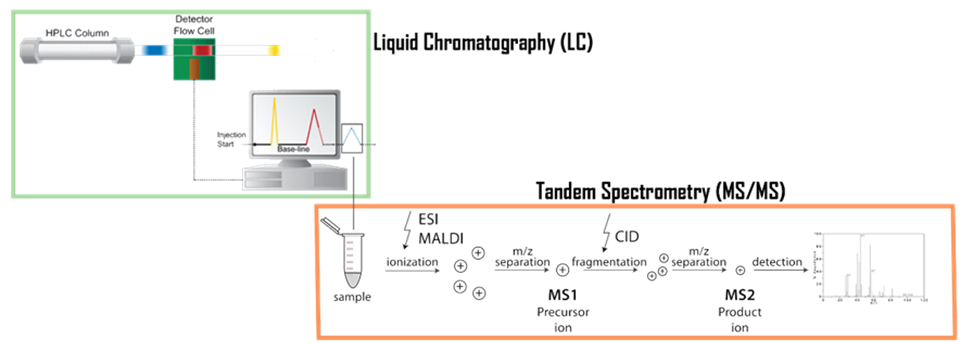
Figure 2. Schema of a tandem mass spectrometry associated with liquid chromatography (LC-MS/MS)
(2) The advantage of LC-MS/MS
LC-MS/MS can analyze small or medium polar molecules with high sensitivity and specificity.
(3) The application of LC-MS/MS
LC-MS/MS can determine the protein molecular weight, analyze peptides and protein modification sites, and identify proteins. It can be used in fields such as biochemistry, agriculture and clinical medicine.
References
- Raquel Pérez-Míguez, María Luisa Marina, María Castro-Puyana. High resolution liquid chromatography tandem mass spectrometry for the separation and identification of peptides in coffee silverskin protein hydrolysates. Microchemical Journal, 2019,149.
- Swearingen Kristian E, Eng Jimmy K, Shteynberg David, et al. A Tandem Mass Spectrometry Sequence Database Search Method for Identification of O-Fucosylated Proteins by Mass Spectrometry. Journal of proteome research, 2019,18(2).

