Untargeted Metabolomics Service
Untargeted metabolomics is a comprehensive approach used to identify and quantify all detectable metabolites in a sample, providing an unbiased analysis of the metabolic profile. This method helps in discovering metabolic changes and potential biomarkers without a predefined focus on specific compounds. At Creative Proteomics, we offer untargeted metabolomics services using advanced LC-MS and GC-MS platforms. These systems enable us to analyze a wide variety of samples, including serum, urine, cerebrospinal fluid, exosomes, and more, allowing for in-depth metabolic profiling and discovery-driven insights.
Submit Your Request Now
×- Services
- Advantages
- Workflow
- Sample Requirements
- Data Analysis
- Demo
- Case
- Platforms
- Database
- Applications
- FAQs
What is Untargeted Metabolomics?
Untargeted metabolomics, namely discovery metabolomics, involves the comparison of the metabolome between the control and test groups, to identify differences between their metabolite profiles which may be relevant to specific biological conditions.The goal of untargeted metabolomics is to capture the broadest range of metabolites in a sample, along with their identification and quantification. Recent advances in instrumentation and computing have enabled the development of this approach.
Untargeted Metabolomics Services
With integrated a set of separation, characterization, identification and quantification systems featured with excellent robustness & reproducibility, high and ultra-sensitivity, Creative Proteomics provides reliable, rapid and cost-effective Untargeted Metabolomics as below.We also provide Targeted Metabolomics Services.
Service Contents
- Serum Untargeted Metabolomics
- Urine Untargeted Metabolomics
- Cerebrospinal Fluid Untargeted Metabolomics
- Plant Untargeted Metabolomics
- Exosome Untargeted Metabolomics
- GC–MS Untargeted Metabolomic Analysis
- LC-MS/MS Untargeted Metabolomics Services
- Gut Flora Metabolomics Research Solution
Serum untargeted metabolomics utilizes NMR, GC-MS, and LC-MS for comprehensive identification and quantification of metabolites, revealing key biological insights.
Urine metabolomics has emerged as a prominent field for non-invasive biomarkers discovery that can detect subtle metabolic changes brought by a specific disease or therapeutic intervention.
Cerebrospinal Fluid Untargeted Metabolomics
With integrated set of separation, characterization, identification and quantification systems featured with excellent robustness & reproducibility, high and ultra-sensitivity, Creative Proteomics provides reliable, rapid and cost-effective CSF untargeted metabolomics.
Learn more about the plant primary metabolism network and plant secondary metabolism network.
Exosome Untargeted Metabolomics
Exosome Metabolomics Service helps you quickly and efficiently get the most information from your exosomes.
GC–MS Untargeted Metabolomic Analysis
The GC-MS untargeted metabolomics developed by Creative Proteomics is an effective method for both qualitative and quantitative analysis of unknown compounds in mixtures.
LC-MS/MS Untargeted Metabolomics Services
Creative Proteomics' LC-MS/MS untargeted metabolomics provides high throughput, high resolution, and high sensitivity for the analysis of metabolites that are difficult to volatilize or have poor thermal stability.
Gut Flora Metabolomics Research Solution
Metabolomic assays are performed on host gut contents or stool samples to study the interaction between the gut flora and the host at the metabolic level.
Why Creative Proteomics?
- Individualized Experimental Protocols: Tailored methods enhance sensitivity, accuracy, and reproducibility in metabolite detection.
- High-Throughput and High-Resolution Capabilities: Enables quantification of 1,000+ metabolites across 12 classes with high-resolution mass spectrometry.
- State-of-the-Art Technical Platform: Advanced LC-MS (MRM) and GC-MS (SIM) Analysis Systems
- Professional Assay and Analysis Capability: Ensures reliable, accurate results with advanced analysis and rigorous quality standards.
Workflow of Untargeted Metabolomics Service
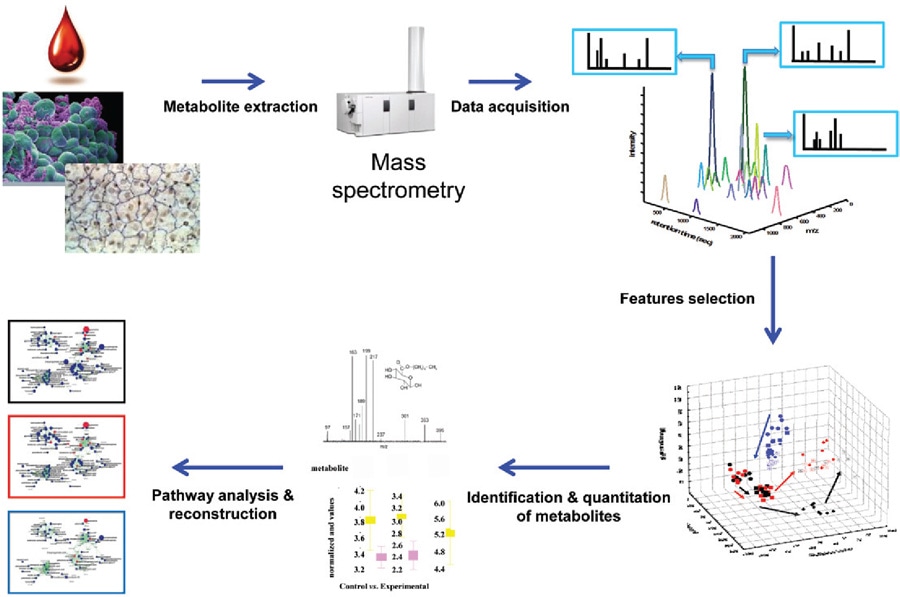
There are usually three steps in the experimental workflow:
1.Profiling in order to seek the metabolites with statistically significant variations in abundance within a set of experimental and control samples;
2.Determination of metabolite ID, including the chemical structure;
3.Comprehensions as the last step which makes connections between the identified metabolites and the biological processes.
In the workflow of discovery metabolomics, analytical reproducibility is critical for expression profiling work; annotation is a tentative identification based on an accurate mass match to a database or a spectral match to a library; the collected data can be interpreted for biomarker discovery, biological signature/fingerprint selection and pathway mapping. Above are the most important parts in untargeted metabolomic research. The tech panel in Creative Proteomics is experienced in sample preparation, data interpretations. It also can provide you reliable metabolite identification.
Sample Requirements
| Sample type | Recommended sample size | Pre-treatment and storage |
|---|---|---|
| Tissue | 100-200 mg | Snap freezing in liquid nitrogen, stored at -80℃. |
| Urine | 200-500 μL | 5000×g 4℃ Centrifuge for 30-60min, remove supernatant, store at -80℃. |
| Serum/plasma | >100 μL | Collected serum/plasma, snap freezing in liquid nitrogen, stored at -80℃. |
| Cerebrospinal fluid, amniotic fluid, bile and other body fluids | >200 μL | 4℃ Centrifuge for 10min, (or filter using 0.22μm membrane), remove supernatant and store at -80℃. |
| Suspension cells | >1*107 | Centrifuge and collect cells after liquid nitrogen snap freezing and store at -80℃. |
| Walled cells | >1*107 | Cultured walled cells are stored in 1.5ml centrifuge tubes, snap freezing in liquid nitrogen and stored at -80℃. |
| Cell supernatant | >2 mL | centrifuge at 4℃ for 3 minutes, take the supernatant and store at -80℃. |
Data Analysis

Demo for Untargeted Metabolomics Service
Figures come from (Li, Y.et.al, Sci Rep,2023)
Untargeted Metabolomics Case study
Technical Platforms
See more Creative Proteomics Mass Spectrometry
The targeted metabolomics service provided by Creative Proteomics is based on our cutting edge separation and analytical platforms. Our experienced technicians have optimized protocols, in order to provide best service to meet customers' requirement.
Database of Metabolites

Applications of Untargeted Metabolomic




Medical Research
Enhances medical research by identifying biomarkers for diseases, understanding pathophysiological mechanisms
Agriculture and forestry field
stress resistance mechanism, breeding ,growth development mechanism and protection research, etc.
Microbiome
pathogenic mechanism, drug resistance mechanism, pathogen-host interaction research , etc.
Biomedicine
mechanism of drug action, drug efficacy evaluation, drug development, etc.
Untargeted Metabolomics FAQs
What are the Advantages of Untargeted Metabolomics?
The advantages of untargeted metabolomics lie in its ability to address the limitations of targeted metabolomics, particularly for discovery-driven and hypothesis-generating studies. Without the need for an exhaustive prior understanding of specific metabolites, untargeted metabolomics can measure thousands of metabolites in a single sample, supporting broad and unbiased metabolic profiling. This approach offers flexibility in sample preparation, can generate extensive quantitative data, and does not require internal standards. Importantly, it enables the detection of both known and unknown metabolites, fostering the discovery of previously unidentified or unexpected metabolic changes.
What databases for metabolite identification?
Public databases such as KEGG, METLIN, etc., have an accuracy of less than 10ppm, and identification can be more accurate if the corresponding secondary spectra are available inside the database. If you want to verify, you can buy standard products. We also supply high quality standard products.
Is there a requirement for the number of samples of untargeted metabolomes?
For untargeted metabolomes whose goal is to find differential metabolites, the number of clinical samples is recommended to be no less than 30 per group. For model organisms and plant and animal samples, no less than 10 are recommended.
What is the difference between untargeted metabolomic analysis and lipid metabolomic analysis?
untargeted metabolome analysis is a research method to quantitatively analyze all metabolites in the organism and find the relative relationship between metabolites and physiological and pathological changes. The research object is small molecular substances with a relative molecular mass less than 1000Da, such as lipids , ketones, organic acids, etc. As we all know, genomics and proteomics explore life activities at the gene and protein levels respectively, but in fact many life activities in cells occur at the metabolite level, such as cell signal release, energy transfer and intercellular communication, etc. are all affected by metabolites regulation. Untargeted metabolome analysis is to discover differentially expressed metabolite information through univariate and multivariate analysis, thereby reflecting the environment in which cells are located and the interaction between them and external factors.
Lipidomics is a discipline that studies the lipid composition, lipid metabolism and lipid interactions of organisms, and is the most important branch of metabolomics. Lipids have a variety of important biological functions, such as material transport, energy metabolism, information transmission, and metabolic regulation. Abnormal lipid metabolism can cause many human diseases, including Alzheimer's disease, diabetes, obesity, and atherosclerosis. hardening, etc.
Learn about other Q&A about other technologies.
Publications
Here are some publications in Metabolomics research from our clients:

- A personalized probabilistic approach to ovarian cancer diagnostics. 2024. https://doi.org/10.1016/j.ygyno.2023.12.030
- Macrophage-associated lipin-1 promotes β-oxidation in response to proresolving stimuli. 2020. https://doi.org/10.4049/immunohorizons.2000047
- A non-probiotic fermented soy product reduces total and ldl cholesterol: A randomized controlled crossover trial. 2021. https://doi.org/10.3390/nu13020535
- Overexpression of maize ZmLOX6 in Arabidopsis thaliana enhances damage-induced pentyl leaf volatile emissions that affect plant growth and interaction with aphids. 2023. https://doi.org/10.1093/jxb/erac522
- Modulation of the tick gut milieu by a secreted tick protein favors Borrelia burgdorferi colonization. 2017. https://doi.org/10.1038/s41467-017-00208-0
Reference
- Li, Yang et al. "Metabolomics-based study of potential biomarkers of sepsis." Scientific reports vol. 13,1 585. 11 Jan. 2023































