Cardiovascular disease is the leading cause of death globally, accounting for 31% of total deaths worldwide according to World Health Organization statistics in 2016. Heart failure (HF) is the final stage of most heart diseases and is commonly referred to as heart decline. Many studies focus on "upstream" omics, such as whole genome sequencing and transcriptome sequencing, revealing information from aspects such as extracellular matrix remodeling, inflammation signals, oxidative stress, mitochondrial dysfunction, and branched amino acid metabolism. However, it is still to be explored whether these upstream changes can be translated into protein levels and further conveyed to downstream metabolites through enzyme levels.
The proteome and metabolome, being the complete sets of proteins and small molecule metabolites respectively, play a crucial role in heart failure research. Changes in the levels of specific proteins or metabolites can indicate abnormal cardiac function and contribute to heart disease.
Case 1 Proteomics study of extracellular matrix remodeling in heart failure (1)
The extracellular matrix (ECM) remodeling is a key pathological feature of heart failure (HF). ECM remodeling is ongoing and leads to systolic and diastolic damage. Genome-wide association studies have shown that ADAMTS family proteases are associated with cardiovascular diseases. ADAMTS5 is one of the most abundant proteases secreted by mouse cardiac fibroblasts. However, the contribution of the ADAMTS5 protease and its substrate versican to heart failure (HF) is unknown. The authors elaborate on the role of ADAMTS5 in ECM remodeling in heart failure and the intervention scheme, which is also the largest-scale heart failure proteomics study to date.
Sample grouping design: ischemic heart failure scar tissue (n=5), non-ischemic heart failure scar tissue (n=10), and healthy heart tissue (From Heart Transplant Donors, n=6). Then, extracellular matrix (ECM) proteinomics was performed. 200 ECM and ECM-related proteins were identified. Accumulation of interstitial proteins, besides fibrillar collagen, was observed in the ischemic heart failure tissue. These members include SLRP (small leucine-rich proteoglycans) family members and the main CSPG (chondroitin sulfate proteoglycans) - proteoglycan VCAN (versican).
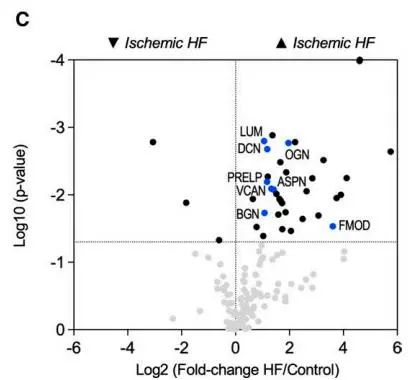
Volcano plot for the proteomics comparison between control (n=6) and nonischemic HF (n=10) patients (GuHCl extracts)
Subsequently, single-cell sequencing showed the specificity of Versican and ADAMTS expression in the human heart. The impact of drug treatment on the composition of ECM in ischemic heart failure was then revealed through large-scale left ventricular proteinomics. A total of 177 ECM and ECM-related proteins were detected in the components of the extracellular matrix, of which 141 proteins could be quantified. Samples with β receptor blockers showed the most proteins with changes in expression. The researchers also found that heart rate was positively correlated with ECM protein abundance, with the strongest correlation being between the abundance of versican protein and heart rate.
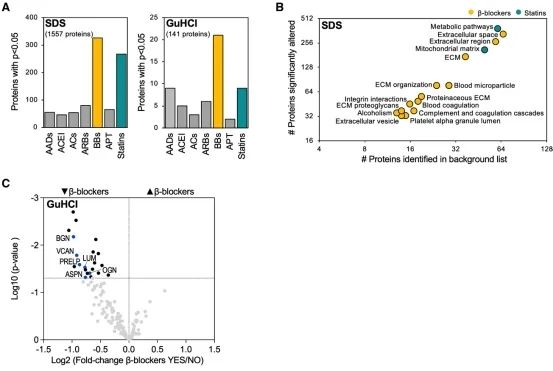
Extracellular matrix remodeling in ischemic heart failure
The researcher in this article confirms that ADAMTS5 cleaves proteoglycans during heart remodeling. In addition, it is demonstrated that proteoglycan accumulation, impaired heart function, and reduced protein involved in intercellular communication exist in mice with Adamts5 activity loss. Finally, the researcher discusses the impact of drugs on extracellular matrix remodeling and reveals that beta-receptor blockade attenuated proteoglycan accumulation in ischemic heart failure.
Case 2 Core functional nodes and sex-specific pathways in human ischaemic and dilated cardiomyopathy (2)
Dilated cardiomyopathy (DCM) and ischemic cardiomyopathy (ICM) are the two main causes of heart failure. This study conducted a comprehensive protein + metabolic screening of human ICM and DCM left ventricular tissues, which were collected in a large number of frozen samples, filling the gap in previous research and finding some common or unique pathways and factors for both diseases, providing new resources for disease diagnosis and treatment target development.
Through the protein + metabolic analysis of ICM and DCM, the study identified 3264 proteins, of which 2614 proteins had quantitative information in at least 33 samples. Compared to healthy hearts, 185 and 377 differential proteins were found in ICM and DCM, respectively, 153 of which were common differential proteins. The serum amyloid A1 (SAA1) had the largest decrease, which was also the first time the protein disturbance was found in human ICM muscle. The expression of many extracellular matrix proteins also changed significantly, which is a well-known feature of heart remodeling. In addition, the change in von Willebrand factor and 5-hydroxytryptamine in ICM suggests the activation of platelets and coagulation cascade, highlighting the mechanism of atherosclerotic thrombosis formation in ICM.
Targeted metabolic analysis found that compared to healthy hearts, there are 13 core regulatory metabolites common to both ICM and DCM hearts, including thyroid hormone, riboflavin 5'-phosphate (also called flavin mononucleotide FMN), purine and pyrimidine nucleotides, etc. These core regulatory metabolites may become new therapeutic targets or biomarkers.
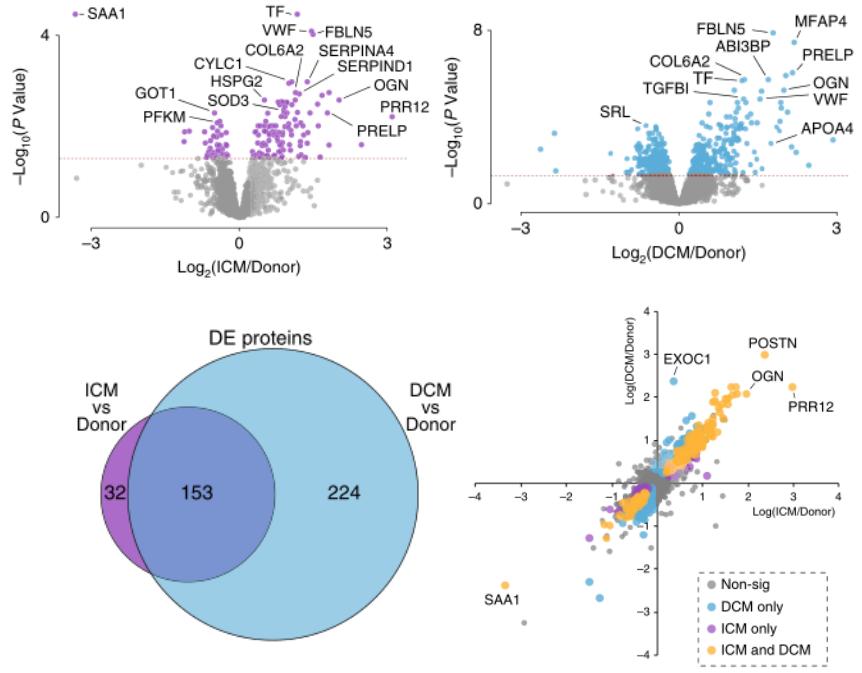
Differential protein analysis of ICM and DCM
The authors further did pathway enrichment analysis using differential proteins and differential metabolites. In the ICM and DCM, nine and ten pathways were found to be regulated at the protein and metabolite levels, respectively. Among them were common pathways such as thyroid hormone synthesis, carbohydrate digestion and absorption, estrogen signalling pathway and aldosterone-regulated sodium reabsorption pathway, which may be the core regulatory pathways in advanced heart failure.
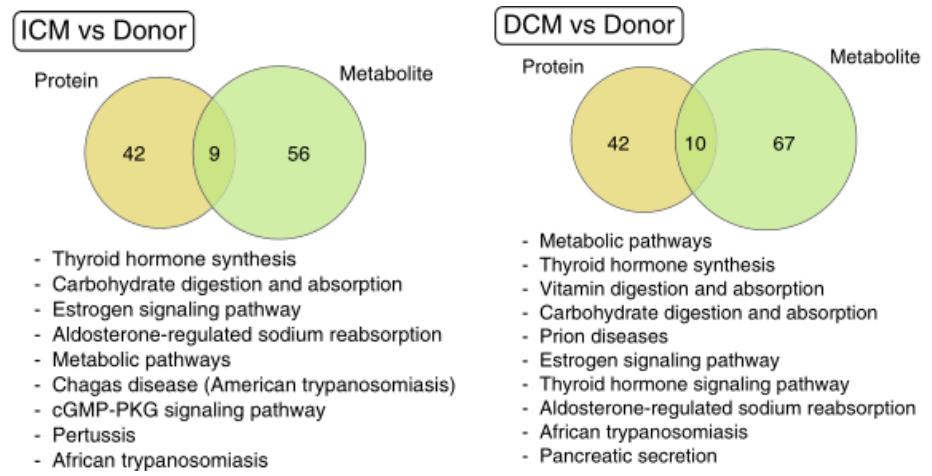
Pathway analysis of ICM and DCM at the protein and metabolite levels
As cardiovascular diseases show clear differences between men and women in terms of clinical presentation, response to treatment and type of heart failure, the researchers finally examined whether proteins and metabolites interacted with gender and identified several new gender interaction factors, such as FMN, short-chain acylcarnitine, ornithine, TMAO and nitric oxide and enzyme inhibitors.
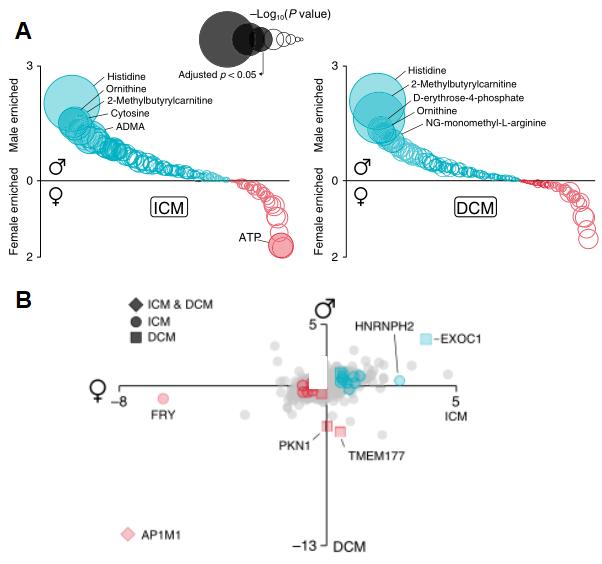
Metabolite-sex interactions plot (A), and protein-sex interactions plot (B)
Reference
- Barallobre-Barreiro, Javier, et al. "Extracellular matrix in heart failure: Role of ADAMTS5 in proteoglycan remodeling." Circulation 144.25 (2021): 2021-2034.
- Li, Mengbo, et al. "Core functional nodes and sex-specific pathways in human ischaemic and dilated cardiomyopathy." Nature Communications 11.1 (2020): 2843.

