Electrophoresis is a major technique for separating proteins and other substances such as nucleic acids, purines, pyrimidines, some organic compounds and even inorganic ions. Most of the current electrophoresis is to separate the sample into the mobile phase in an immobilized medium. Polyacrylamide gel is one of the main media. It is a porous gel whose pore size is close to the size of protein molecules, which improves the resolution of proteins. Moreover, the polyacrylamide gel has good chemical stability, strong repeatability, stability to changes in pH and temperature, and easy color observation. SDS polyacrylamide gel electrophoresis (SDS-PAGE) has the advantages of simple operation and good reproducibility in the determination of protein molecular weight, detection of specific proteins, and identification of strain species.
The Principle of SDS-PAGE
Polyacrylamide gel is composed of acrylamide and cross-linking agent N, N'-methylenebisacrylamide under the action of catalysts ammonium persulfate (AP) and N, N, N', N'-Tetramethylethylenediamine (TEMED). It is a gel with a three-dimensional network structure. PAGE can separate proteins into several bands according to the different mobility caused by the different charge and molecular weight of protein molecules. SDS is an anionic surfactant, which can break the hydrogen and hydrophobic bonds of proteins in the presence of reducing agents (β-mercaptoethanol or dithiothreitol, DTT), and combine with protein molecules in a certain ratio to form short rod-shaped composites of the same density. Positively correlated with the molecular weight of the protein, the length of the complex formed by proteins of different molecular weights is different. SDS makes the amount of negatively charged protein far exceed its original charge, masking the natural charge difference between various protein molecules. Therefore, the mobility of various protein-SDS complexes during electrophoresis is no longer affected by the original charge and molecular shape, but only depends on the relative molecular mass.
 The polyacrylamide gel is usually composed of a stacking gel in the upper layer and a separating gel in the lower layer. The difference between the upper and lower gels is the concentration of acrylamide and the pH of Tris-HCl. During electrophoresis, an electric field is applied to the gel, and negatively charged proteins migrate across the gel from the negative electrode to the positive electrode. The most common electrophoresis buffer consists of Tris and glycine. The pH in the stacking gel is 6.8, and only a few glycine molecules dissociate. Therefore, the SDS-treated protein molecules move between the upper glycine molecule and the lower Cl- ion. This process compresses the protein sample in the gel into bands that are much smaller than the volume initially loaded. As the electrophoresis progresses, the protein moves to the separating gel (pH 8.8), where of the glycine molecules dissociate. The speed of the movement increases and exceeds the protein. In the separating gel, the speed of movement of each protein depends on its molecular weight. Proteins with small molecular weights can pass through the pores in the gel easily, while those with large molecular weights have more difficulty passing through. After a period of time, proteins reach different distances according to the sizes, achieving the purpose of protein separation.
The polyacrylamide gel is usually composed of a stacking gel in the upper layer and a separating gel in the lower layer. The difference between the upper and lower gels is the concentration of acrylamide and the pH of Tris-HCl. During electrophoresis, an electric field is applied to the gel, and negatively charged proteins migrate across the gel from the negative electrode to the positive electrode. The most common electrophoresis buffer consists of Tris and glycine. The pH in the stacking gel is 6.8, and only a few glycine molecules dissociate. Therefore, the SDS-treated protein molecules move between the upper glycine molecule and the lower Cl- ion. This process compresses the protein sample in the gel into bands that are much smaller than the volume initially loaded. As the electrophoresis progresses, the protein moves to the separating gel (pH 8.8), where of the glycine molecules dissociate. The speed of the movement increases and exceeds the protein. In the separating gel, the speed of movement of each protein depends on its molecular weight. Proteins with small molecular weights can pass through the pores in the gel easily, while those with large molecular weights have more difficulty passing through. After a period of time, proteins reach different distances according to the sizes, achieving the purpose of protein separation.
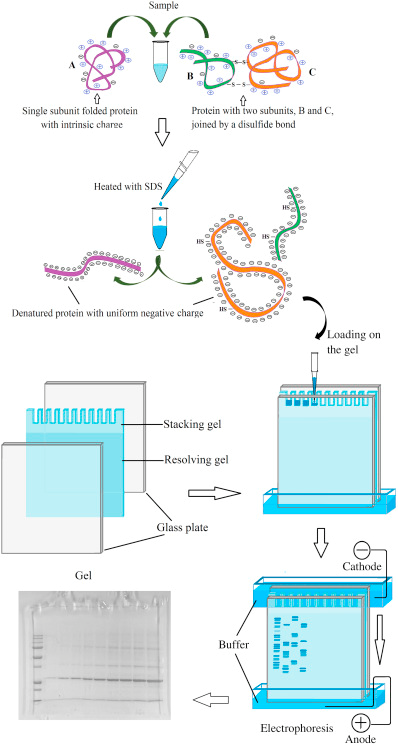
Figure 1. Schematic diagram of polyacrylamide gel electrophoresis (Gülay, et al, 2018).
How to Determine Molecular Weight of Protein by SDS-PAGE?
SDS-PAGE is the main method to determine the molecular weight of unknown proteins. A protein with known molecular weight and an unknown sample are electrophoresed at the same time. After staining, according to the relative mobility of the standard protein and the logarithm of the molecular weight, a line can be obtained and determine the molecular weight of the unknown sample using its relative mobility. In the laboratory, a standard molecular weight protein covalently coupled to a dye is used as a reference protein to roughly indicate the size of the unknown protein. This pre-stained protein marker can be directly observed during electrophoresis or when transferring membranes.
How to Read SDS-PAGE Results?
After electrophoresis, protein separation cannot be directly observed by the naked eye, and subsequent staining techniques are needed. Coomassie brilliant blue staining and silver staining are common methods for routine detection and quantification of proteins separated by electrophoresis. After simple processing such as fixation-staining-decolorization, the distribution of protein can be clearly observed. With the improvement of high-sensitivity protein analysis methods and protein identification technologies, new staining methods such as fluorescent labeling and isotope labeling technology have greatly improved sensitivity, and are also compatible with automated proteome platform gel cutting technology. More high sensitivity and automated dyeing technologies are been developed.
How to Store SDS-PAGE Gel?
Freshly SDS-PAGE gels are usually prepared before each experiment. However, gels can also be stored in clean water at 4°C for about a week. If the gel cannot be photographed in time after dyeing, it needs to be placed in water to prevent drying and shrinking of the gel. It is advised to photograph the staining results as soon as possible. Band will disperse if the gel is soaked in water for a long time.
What Our SDS-PAGE Service Provide
Creative Proteomics is a leading provider of SDS-PAGE analysis services that leverages LC-MS/MS technology to provide accurate and efficient identification of proteins in SDS-PAGE and 2-DE samples. With an advanced in-gel digestion method, we significantly improve the success rate of protein mass spectrometry identification and peptide coverage.
Our service offers high sensitivity, versatility, and throughput, with the ability to identify tens to hundreds of proteins at a time. Operating with liquid phase coupled with mass spectrometry, our automated approach enables fast analysis and optimal separation.
References
- Smith B J. SDS Polyacrylamide Gel Electrophoresis of Proteins. Methods in Molecular Biology, 1984, 1(4):41-55.
- Duffy M F, Noormohammadi A H, Baseggio N, et al. Polyacrylamide gel-electrophoresis separation of whole-cell proteins. Methods in Molecular Biology, 1998, 104:267.

