What Is Polysaccharide NMR Analysis and What Can It Reveal?
Polysaccharide Nuclear Magnetic Resonance (NMR) analysis enables precise structural decoding of carbohydrate polymers by monitoring chemical shifts, scalar couplings, and through-space interactions. Through tailored 1D (1H, 13C, DEPT) and 2D (COSY, HSQC, HMBC, NOESY) experiments, we map residue identities, determine α/β anomeric configurations, and assign glycosidic linkage positions. The platform resolves branching architectures, substitution sites (e.g., acetyl, sulfate, phosphate), and even aggregation behavior via DOSY profiling. These insights inform structure–function studies, batch release, and process optimization in food, plant, and microbial polysaccharides.
Services We Provide — Polysaccharide NMR & Spectrum Interpretation
At Creative Proteomics, we transform complex polysaccharide matrices into interpretable, decision-ready data using a modular NMR platform. Our services are designed around your research objectives and material characteristics, whether you're validating structure, comparing batches, or investigating functional properties.
Residue Identification & Linkage Assignment
- Identify sugar residues and assign α/β anomeric configuration
- Determine glycosidic linkage positions (→2, →3, →4, →6)
- Resolve branch points and side-chain substitutions
Branching, Substitution & Structural Complexity Mapping
- Localize O-acetylation, O-sulfation, and phosphorylation sites
- Quantify acetyl or methyl substitution via 1H integration and HMBC correlation
- Detect branching proximity via NOESY/ROESY inter-residue interactions
Size Distribution, Conformation & Aggregation State
- Characterize sample heterogeneity and aggregation behavior using DOSY
- Assess temperature-sensitive dispersion through VT-NMR
- Detect low-molecular-weight impurities in partially degraded samples
Comparability & Quality Testing
- Spectrum overlay and deviation metrics for batch comparability or raw material validation
- Optional integration with SEC-MALS or FT-IR for release testing support
Optional Add-On Services for Structural Confidence
- Monosaccharide Composition Analysis – with uronic-acid reduction for accurate linkage confirmation
- Methylation Linkage Profiling – for cross-validation of NMR-derived structures
- SEC-MALS – for molar mass and size distribution aligned with DOSY
- FT-IR/Raman – for functional group validation and rapid screening
- Integrated Structural Reports – combine NMR, PMAA, and SEC-MALS for unified insight
Whether you're confirming a known structure or exploring a new material, we tailor every NMR experiment set to your analytical endpoint.
How We Analyze Polysaccharides Using NMR
Polysaccharide NMR Methods and 2D Experiments We Offer
- 1D Surveys: 1H, 13C, DEPT-135 for residue and backbone signals
- 2D Through-Bond: COSY/TOCSY (spin systems), HSQC/HSQC-TOCSY (fingerprints), HMBC (linkages)
- 2D Through-Space & Dynamics: NOESY/ROESY (branching, proximity), DOSY (size, aggregation)
- Optional: 31P NMR (phosphorylation), 19F NMR (fluorinated probes), selective 1D/2D for crowded spectra
Acquisition Settings and NMR Parameters for Polysaccharide Analysis
- Field Strength: 400–900 MHz; cryoprobe for low-concentration samples
- Resolution Control: anomeric 1H line width target ≤ 1.2 Hz
- Solvents: D2O, DMSO-d6, pyridine-d5 matched to sample type
- Water Suppression: presaturation, excitation sculpting, or gradient-based methods
- Temperature: standard 298 K; adjustable to 353 K; VT-NMR for sensitive samples
- Referencing: TSP or acetone for 1H; external refs for 31P and 19F
- 2D Matrix: typically 1024 × 256–512 points, optimized per dataset

Quality Control, Reproducibility, and Troubleshooting
- Shimming and line-width targets documented for anomeric regions; repeat checks on critical planes.
- Solvent-suppression strategy validated to avoid saturation near exchangeable protons.
- Artifact monitoring: t1-noise, axial peaks, phase-roll; corrective reprocessing applied where needed.
- Traceability: pulse programs, delays, scans, receiver gain, temperature logs included in the report.
- Mitigations: ionic-strength tuning, chaotropes within reason, temperature ramps, or DOSY to manage aggregation.
Step-by-Step Workflow for Polysaccharide NMR Analysis
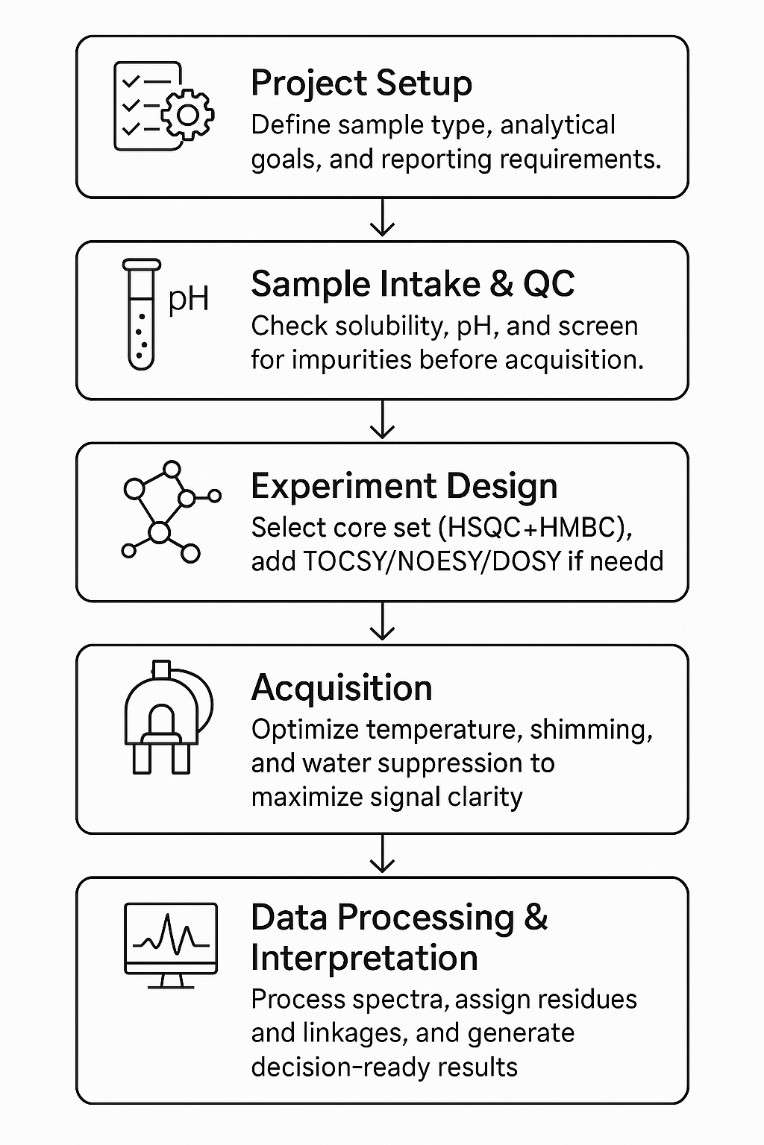
Deliverables: Annotated Spectra, Assignments, Linkage Maps
Our service provides decision-ready results with all key outputs required for structural interpretation and quality evaluation:
- Annotated 1D/2D spectra – fully labeled with residue and linkage assignments
- Residue-level assignment tables – 1H/13C chemical shifts and correlation summaries
- Linkage and branching maps – graphical representation of glycosidic connections
- Comparability overlays – batch-to-batch or supplier evaluation with deviation metrics
- Method sheet – acquisition conditions, solvent system, and referencing details for reproducibility
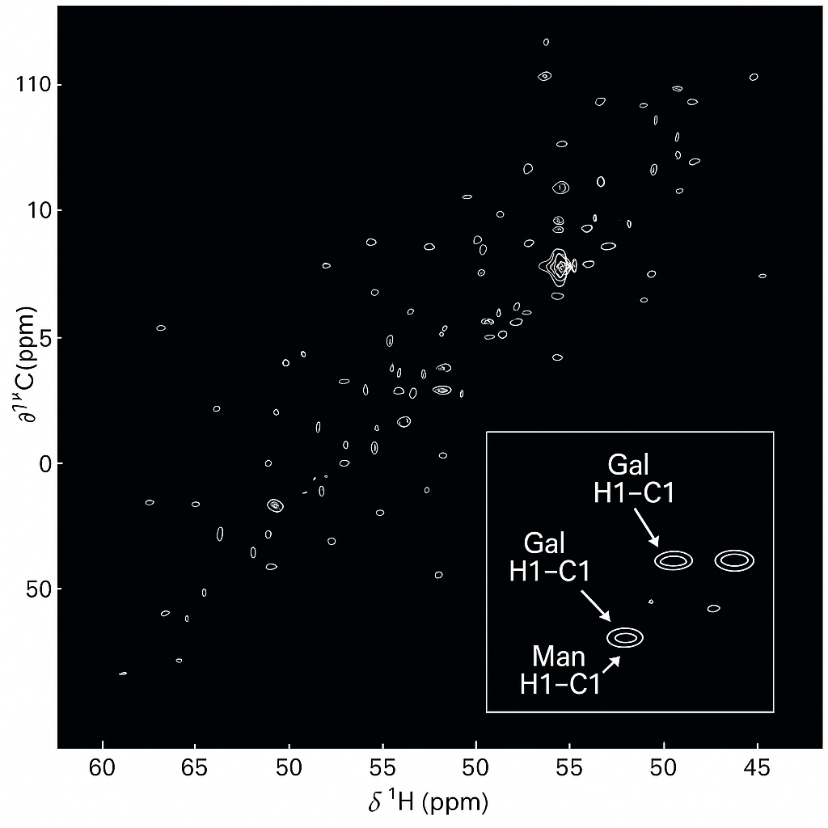
Composite HSQC + Anomeric Zoom
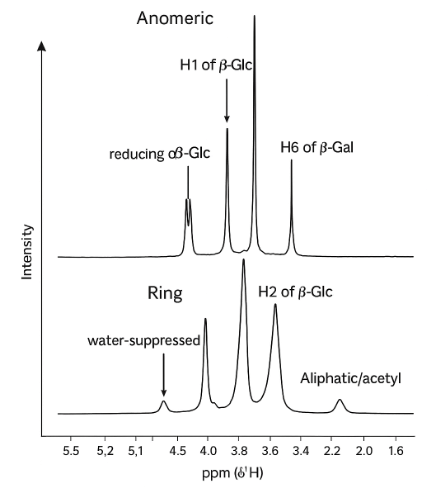
1D 1H NMR Spectrum
How Should You Prepare and Submit Your Samples?
| Sample Type | Recommended Amount | Preferred Form | Typical Solvent | Submission Notes |
|---|---|---|---|---|
| Neutral polysaccharides | ≥30 mg (powder) or 0.6–1.0 mL @ 10–30 mg/mL | Lyophilized powder or D2O solution | D2O | Desalting recommended; avoid surfactants or protein residues. |
| Uronic acid–rich / acidic polysaccharides | ≥35 mg or 0.8 mL @ 15–30 mg/mL | Powder preferred | D2O (Na+ form) | Exchange to sodium form to prevent peak broadening; note pH. |
| Sulfated / heavily substituted polysaccharides | ≥40 mg or 0.8 mL @ 20–40 mg/mL | Powder preferred | D2O or D2O/DMSO-d6 | May require mixed solvents due to poor solubility; indicate sulfation degree. |
| Viscous gels / partially insoluble samples | ≥50 mg or 1.0 mL @ 20–50 mg/mL | Powder strongly preferred | DMSO-d6 or pyridine-d5 | Provide any heating or co-solvent instructions; avoid sample degradation. |
| Oligosaccharides (DP ≤ 10) | 5–15 mg or 0.6 mL @ 2–10 mg/mL | Powder or D2O solution | D2O | Ideal for 1D/2D fingerprinting; cryoprobe available for low-mass samples. |
| Isotopically labeled or modified | 10–20 mg or 0.6 mL @ 5–15 mg/mL | Powder or solution | D2O (1H/13C), external reference for 31P/19F | Indicate labeling type; 31P and 19F NMR available upon request. |
| Crude polysaccharide extracts | ≥60 mg or 1.0 mL @ 30–50 mg/mL | Powder preferred | TBD | Prior desalting/deproteinization strongly recommended; provide processing history. |
| Formulations in buffer or saline | 1.0 mL @ 20–50 mg/mL | Solution | D2O or mixed aqueous | Specify buffer/salt type and pH; excessive salt may affect resolution. |
Use Cases – When to Choose Polysaccharide NMR Analysis
![]()
Food and Nutraceuticals
Verify structure–function relationships in bioactive polysaccharides (e.g., β-glucans, arabinogalactans). NMR confirms backbone linkages, side-chain patterns, and consistency across production lots—supporting health claims and QC documentation.
![]()
Plant and Agricultural Research
Characterize pectin (HG, RG-I, RG-II), hemicellulose (XG, arabinoxylan), and callose structures. NMR assists in identifying structural domains, crosslinking motifs, and cell wall remodeling in response to stress or genetic modifications.
![]()
Microbial and Marine Polysaccharides
Analyze EPS, alginates, fucoidans, and sulfated glycans. NMR enables accurate profiling of substitution (acetyl, sulfate), monomer sequences, and chain flexibility, essential for viscosity control, fermentation optimization, or pharmaceutical development.
![]()
Biopharmaceuticals and Drug Delivery
Support the development of polysaccharide-based hydrogels, conjugates, and adjuvants. NMR helps confirm substitution ratios, crosslinkable sites, and batch integrity, ensuring consistent bio-performance and regulatory readiness.
![]()
Synthetic and Engineered Polysaccharides
Evaluate structural fidelity of chemically modified or biosynthetically engineered glycans. NMR provides linkage confirmation, detects side reactions, and offers fingerprinting for comparability protocols or method validation.
Frequently Asked Questions
Q1. Can NMR confirm that my polysaccharide matches a proposed structure?
Yes—choose NMR when you need residue-level verification of backbone identity, anomeric configuration, and linkage topology to accept/reject a proposed structure.
Q2. When should I use NMR to differentiate branching versus random substitution?
Use it when side-chain location and density affect performance (e.g., solubility, bioactivity). NMR resolves branch points and distinguishes substitution on specific ring positions.
Q3. Can NMR tell whether two lots are materially the same?
Yes. NMR is well-suited for lot comparability; it highlights subtle structural shifts and minor contaminants that other assays may miss.
Q4. Should I run NMR after chemical or enzymatic modification (e.g., sulfation, oxidation)?
Yes—NMR detects targeted changes while checking that the backbone remains intact, enabling go/no-go decisions for process optimization.
Q5. Why is my sample viscous or forming gels—can NMR help?
NMR readouts of conformation and aggregation support root-cause analysis for unexpected viscosity or gelation and guide formulation adjustments.
Q6. Is NMR appropriate for authenticity or identity testing of commercial polysaccharides?
Yes. Use it to build a spectral fingerprint for rapid identity checks and to differentiate supplier sources or grades.
Q7. When does NMR add value over composition-only methods?
When linkage type, branching pattern, or substitution position—not just monomer ratios—drive function or quality decisions.
Q8. Can NMR evaluate sulfation/acetylation levels in marine or microbial glycans?
Yes. NMR provides site-specific evidence for the presence and distribution of sulfate/acetyl groups and supports semi-quantitative assessments.
Q9. We engineer plant cell-wall polysaccharides (HG, RG, XG). Is NMR the right tool?
Yes—choose NMR to map domain motifs and cross-link features that correlate with mechanical properties or processing outcomes.
Q10. Do I need NMR for impurity or residual-reagent risk assessment?
Use it when small organic residues or unintended side products may co-exist with the polymer; NMR can reveal low-level species that impact safety or performance.
Q11. Can NMR support comparability for fermentation or biosynthetic processes?
Yes. It provides a structural fingerprint to monitor drift across process changes, scale-ups, or supplier transfers.
Q12. When should NMR be combined with orthogonal tests?
Combine NMR with orthogonal analytics when decisions require cross-validated structure (e.g., regulatory dossiers, high-value materials). NMR supplies the positional/structural context that complements other readouts.


