Comprehensive glycosylation characterisation of glycoproteins is required by the ICH Q6B guidelines. O-linked glycosylation services are offered by Creative Proteomics to help clients in other countries better comprehend how proteins work and regulate, as well as their crucial role in disease and scientific research.
O-linked Glycosylation
O-linked glycosylation, a post-translational alteration of proteins, involves the attachment of specific serine or threonine residues to glycans, or carbohydrate molecules. Enzymes called glycosyltransferases catalyze the transfer of sugar molecules from the sugar donors in nucleotides to the hydroxyl groups in amino acids, mediating this process.
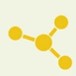
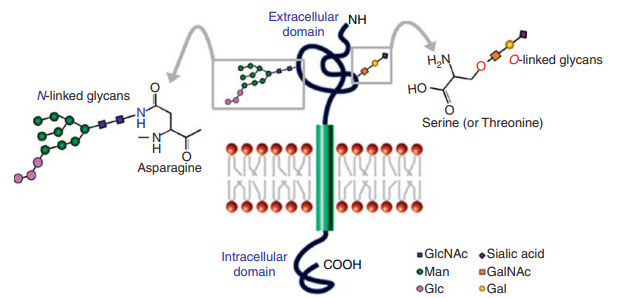 Fig 1. N-linked and O-linked glycosylation on plasma membrane-bound proteins. (Wang, Y.-C., et al.; 2015)
Fig 1. N-linked and O-linked glycosylation on plasma membrane-bound proteins. (Wang, Y.-C., et al.; 2015)
While N-linked glycosylation takes place at the endoplasmic reticulum (ER) and involves the attachment of sugars to asparagine residues, O-linked glycosylation often takes place at the Golgi, changing serine or threonine residues. The glycans attached may differ in content and structure depending on the presence of particular glycosyltransferases, making this process more diverse and changeable than N-linked glycosylation.
Application of Glycoconjugates
- Pluripotency Signaling Modulation
Glycoconjugates can affect the signaling pathways involved in maintaining pluripotency and self-renewal of embryonic stem cells. The addition or removal of specific sugar moieties to glycoproteins or glycolipids can modulate their interactions with ligands, receptors, and other signaling molecules. This, in turn, can influence the activation or inhibition of pluripotency-related signaling pathways, such as the Wnt, Notch, and TGF-β pathways.
- Biomarkers for Cellular Pluripotency and Differentiation
Glycoconjugates help regulate cell signaling, adhesion, and recognition events. Glycosyl conjugates serve as biomarkers for assessing cellular totipotency and differentiation status. Changes in glycosylation patterns of specific proteins can indicate the transition from a pluripotent to a differentiated state. By analyzing the glycosylation profiles of specific glycoproteins or glycolipids, researchers can gain insights into the progression of cellular differentiation, thereby monitoring and characterizing cellular pluripotency and differentiation processes.
- Extracellular Matrixfor (ECM) Cell Culture
Glyconjugates modify cell signaling pathways, regulate cell adhesion, and provide structural support for cells in the ECM. Glyconjugates help cells move, keep them looking the way they should, and regulate their activity. Lyconjugates in the ECM also promote cell development and differentiation by binding to growth hormones and cytokines and creating reservoirs for their controlled release.
- lmmunogenicity of Differentiated Derivatives for Cell Therapy
Glycoconjugates are extensively researched in the context of differentiated derivatives for cell therapy and play a significant influence in immunogenicity. By engaging with particular receptors on immune cells, glycoconjugates can behave as antigens and elicit an immunological response. For differentiated derivatives to be safe and effective in cell therapy, it is crucial to understand how immunogenic they are. In order to limit immunogenicity and enhance the therapeutic effects of cell treatments, glycoconjugate analysis helps to detect potential immunogenic epitopes.
 Fig 2. Glycoconjugates may provide potential solutions for four critical issues in stem cell biology and regenerative medicine. (Wang, Y.-C., et al.; 2015)
Fig 2. Glycoconjugates may provide potential solutions for four critical issues in stem cell biology and regenerative medicine. (Wang, Y.-C., et al.; 2015)
What can We Offer?
Lectin-based detection: Lectins are proteins that can specifically bind to carbohydrate structures. By using lectins that have affinity for O-linked glycans, you can visualize glycosylated proteins. Lectins like concanavalin A, wheat germ agglutinin, and peanut agglutinin can be used.
Periodic Acid-Schiff (PAS) staining: This method involves using periodic acid to selectively oxidize the glycosidic linkages in glycans, followed by treatment with Schiff's reagent. This results in a color reaction that can be visualized using light microscopy.
Gel Electrophoresis: Separating proteins using gel electrophoresis, such as SDS-PAGE, can help detect O-linked glycosylation. After electrophoresis, the gel can be stained with glycoprotein-specific stains, such as Pro-Q Emerald stain or glycoprotein-specific antibodies.
Mass Spectrometry: Mass spectrometry (MS) can be used to identify and characterize O-linked glycosylation sites by analyzing glycopeptides. MS techniques like MALDI-TOF/TOF or ESI-MS/MS can provide information about the glycan structure attached to specific amino acid residues.
Antibody-based detection: Antibodies specific to O-linked glycans or to the modified protein can be used for detection. Techniques like Western blotting or immunofluorescence can be employed using appropriate antibodies to visualize the presence of O-linked glycans.
Service Workflow
Our Goals
Leading experts in O-linked glycosylation analysis include Creative Proteomics. We have a group of seasoned experts with expertise in glycoproteomics who are proficient in cutting-edge analytical methods. Also included in the extensive list of glycoproteomics services we provide are analyses of N-linked glycosylation, glycosylation site occupancy, and glycopeptides. Contact us if you would like further details.
Reference
- Wang, Y.-C., et al.; The "sweet" spot of cellular pluripotency: protein glycosylation in human pluripotent stem cells and its applications in regenerative medicine. Expert Opinion on Biological Therapy. 2015, 15(5), 679–687.
Related Sections
Services
Applications
For research use only, not intended for any clinical use.

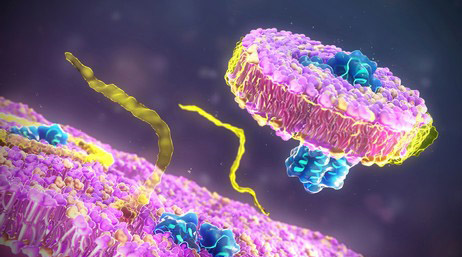
 Fig 1. N-linked and O-linked glycosylation on plasma membrane-bound proteins. (Wang, Y.-C., et al.; 2015)
Fig 1. N-linked and O-linked glycosylation on plasma membrane-bound proteins. (Wang, Y.-C., et al.; 2015) Fig 2. Glycoconjugates may provide potential solutions for four critical issues in stem cell biology and regenerative medicine. (Wang, Y.-C., et al.; 2015)
Fig 2. Glycoconjugates may provide potential solutions for four critical issues in stem cell biology and regenerative medicine. (Wang, Y.-C., et al.; 2015)