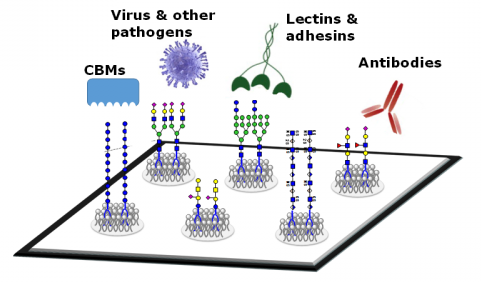
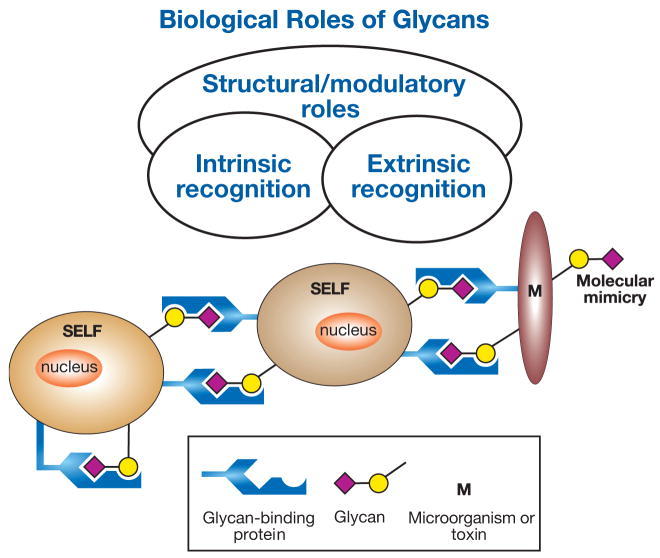
Glycans are defined as the compounds that consist of a large number of monosaccharides linked glycosidically. Glycans have diverse biological functions and it can be classified into two broad categories. One is the structural and modulatory properties of glycans and the other is the specific recognition of glycans by other molecules, most commonly, glycan-binding proteins (GBPs). The GBPs can be divided into two types, intrinsic GBPs and extrinsic GBPs. The intrinsic GBPs recognize glycans from the same organism and mediate cell-cell interactions or recognize extracellular molecules. The extrinsic GBPs which contain pathogenic microbial adhesins, agglutinins, or toxins, recognize glycans from different organisms and some also mediate symbiotic relationships.
Figure 1. General classification of the biological roles of glycans. (Varki A., & Lowe J B; 2009)
Due to the complexity of glycan molecules, glycomics has its own characteristics. First, glycans have a high degree of structural specificity. Second, since the synthesis of glycans is not guided by the template, it is formed under the action of various forms of enzymes, and even in the same glycosylation site of the same kind of molecule, the structure of the carbohydrate chain is also different. In addition, the interaction between glycans and proteins is accomplished through the multivalent and intensifying affinity between them, and this force is relatively weak. These characteristics place very high demands on analytical techniques. Therefore, in order to carry out research on the structure and function of glycans, researchers have established a new research method - glycan microarray. Glycan microarrays first described in 2002 provide a high-throughput means of profiling the interactions of glycan-binding proteins with their ligands. This method can be used to identify specific binding of glycans by lectins, antibodies, toxins, viruses, etc.
The principle of glycans microarray
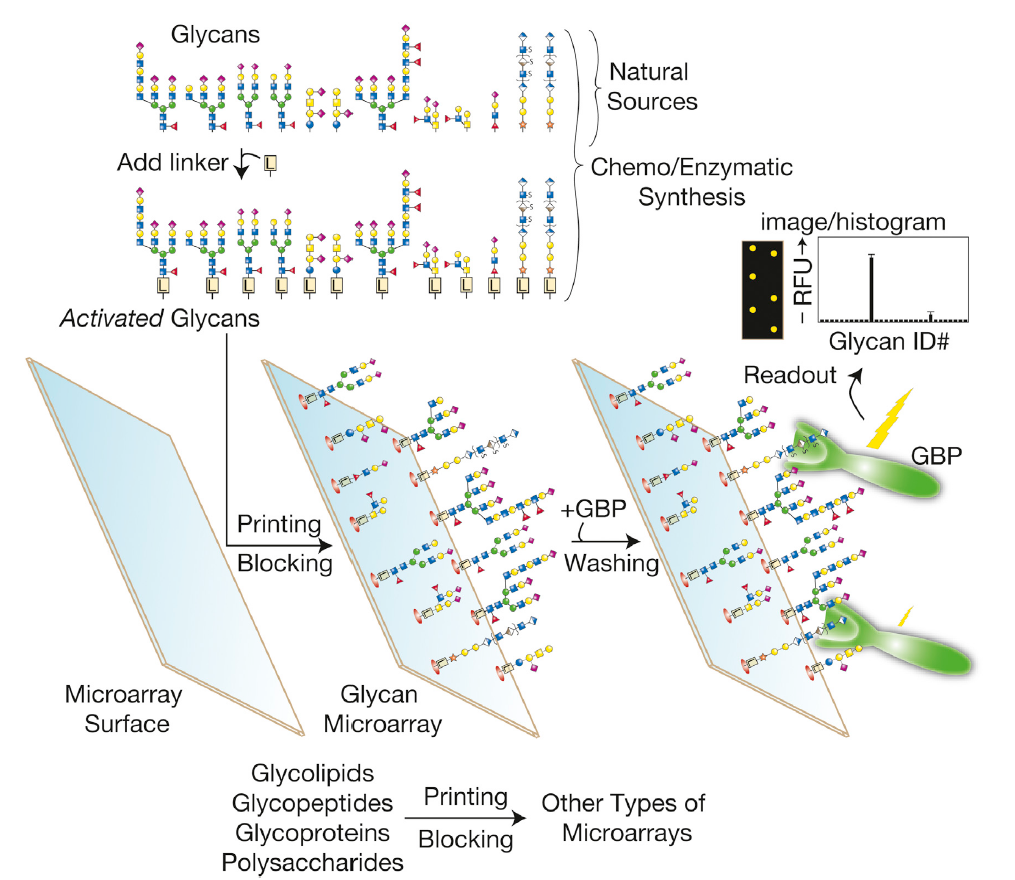
Figure 2. The produce of glycan microarrays. (Cummings R D, Pierce J M;2014)
In glycan microarrays, individual glycans which are from natural sources or chemo or enzymatic syntheses are modified to allow themselves covalently or non-covalently to attach to a solid surface. The glycan microarray can be interrogated with GBPs or other reagents or even cells and viruses. Through this way, those recognized glycan ‘‘spots’’ are identified, which can be visualized by either direct or indirect fluorescent tagging. As shown in Figure 2, the results indicate that the GBPs bound strongly to one glycan, less strongly to another glycan, and did not bind appreciably to any other glycan. Such microarrays can also be prepared from glycolipids, glycopeptides, whole glycoproteins, or polysaccharides.
Applications of glycan microarrays in Glycomics.
Various applications of glycan microarrays have been made since the first report in 2002.
- Rapid profiling of glycan binding properties of proteins, including plant and animal lectins, antibodies, and growth factors. To achieve the goals, carbohydrate microarrays are incubated with fluorophore-labeled GBPs, fluorophore-labeled secondary reagents that interact with the GBPs, or a tag (like biotin or His tag) conjugated to the GBPs. And then a high-resolution fluorescence microarray scanner is applied to quantify the fluorescence intensity of the bound proteins. In addition, surface plasmon resonance (SPR) imaging and mass spectrometry (MS) techniques are employed as label-free detection methods in glycan microarrays.
- Examine glycan binding properties of whole pathogens and to detect pathogens as part of diagnoses. Many pathogenic bacteria display the pathogenic properties, which are caused by the binding of pathogens to host cell surface glycans through specific glycan−protein interactions. The researchers incubated a microarray containing glycans with the Escherichia coli strain, which was stained with the fluorescent dye. The microspots containing mannose epitopes exhibited fluorescence because of the mannose-binding protein on pili expressed by the strain. In order to make this approach become widespread, experimental conditions employed for this purpose should be optimized.
- Determine the quantitative binding affinities of proteins to glycans. Researchers applied glycan microarrays to determine concentrations (IC50) of soluble inhibitors to inhibit 50% of protein binding to glycans. In this assay, a series of pre-incubated mixtures of the fluorophore-labeled lectin and an inhibitor are applied to glycan microarrays. The IC50 values of the inhibitors are then determined by measuring the fluorescence intensities of bound proteins that remain on the microarrays after washing.
- Employ the microarray technology for rapid screening of functional glycans that elicit cellular responses by interacting with cell-surface lectins. Carbohydrate microarrays are constructed by attaching unmodified glycans to the hydrazide-coated surface. Mammalian cells expressing lectins are pretreated with a fluorescent probe, PF1. PFI can selectively detect reactive oxygen species (ROS). The binding of glycan ligands to lectins on cell surfaces can trigger a cellular response that results in the generation of ROS. Therefore, the resulting fluorescence signal emanating from PF1 is directly related to glycan binding to cell-surface lectins. And the microarrays containing glycans are incubated with these mammalian cells. The functions are identified by measuring the fluorescence intensity of each spot. The results show that cells that adhere to glycans on the microarrays generate ROS, as reflected in an increase in fluorescence signals induced by the ROS probe.
References:
- Varki A, Lowe J B. Biological roles of glycans. 2009.
- Hyun J Y, Pai J, Shin I. The Glycan Microarray Story from Construction to Applications[J]. Accounts of chemical research, 2017, 50(4): 1069-1078.
- Cummings R D, Pierce J M. The challenge and promise of glycomics. Chemistry & biology, 2014, 21(1): 1-15.