One-carbon metabolism, mediated by the folate cofactor, is a group of biochemical reactions with a special set of enzymes and coenzymes. It is referred to as one-carbon metabolism because what they have in common is the transfer of one-carbon groups. One-carbon metabolism plays an important role in multiple physiological processes, including biosynthesis (purines and thymidine), amino acid homeostasis (glycine, serine, and methionine), epigenetic maintenance, and redox defense.
One-
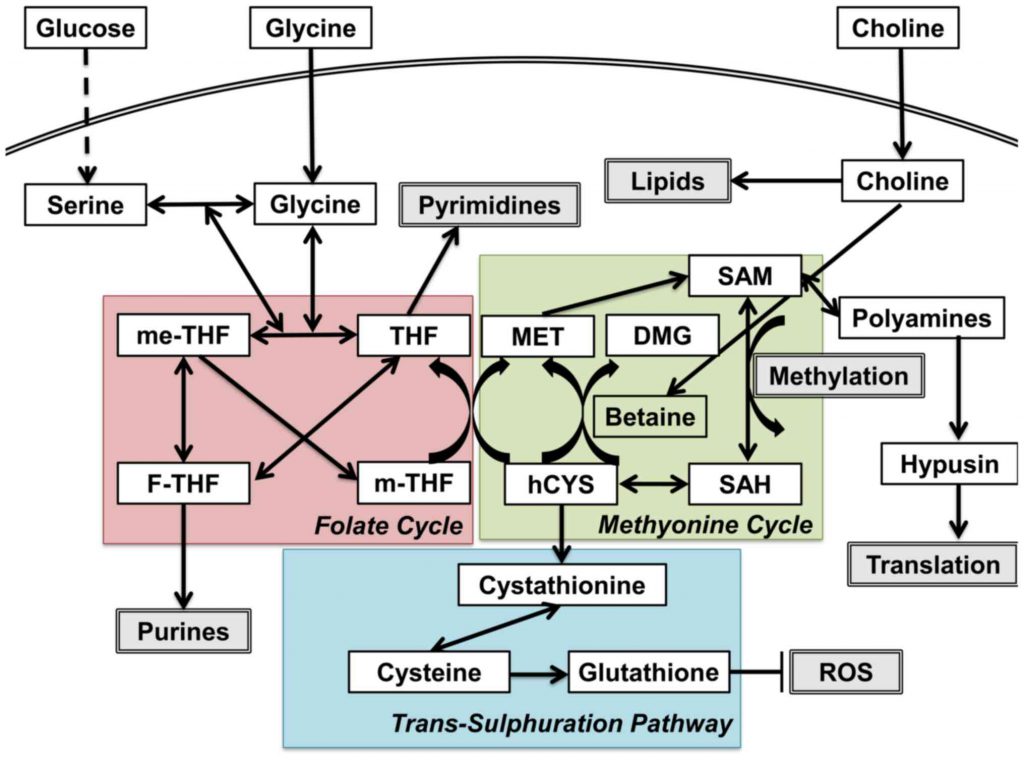
One-carbon metabolism contains three critical reactions: the folate cycle, the methionine cycle, and the trans-sulfuration pathway. In the folate cycle, glycine and serine fuel mitochondrial enzymes through purine production. In the methionine cycle, S-
Source of carbon
One-carbon pool refers to the pool of the methyl groups that are available for the methylation of a variety of compounds, including protein, DNA, RNA, etc. Serine, glycine, formate, histidine, and the choline-derived methyl-glycine species (sarcosine and dimethylglycine) can all directly contribute to the tetrahydrofolate (THF)-bound one-carbon pool. Among them, only three (histidine, serine, and formate) can directly feed into the cytosolic folate-bound one-carbon pool. In the mitochondrial, one-carbon units can be made from serine, glycine, sarcosine, or dimethylglycine and excreted into the cytosol as formate. Histidine degradation generates 5-
One carbon metabolism in cancer
One-carbon units can be used for nucleotide synthesis, methylation, and reductive metabolism, which support the high proliferative rate of cancer cells. Aminoacids (like serine) are major one-carbon sources, and cancer cells are particularly susceptible to the deprivation of one-carbon units by serine restriction or inhibition of de novo serine synthesis. In tumor cells, the processes of cell growth and proliferation require the construction of building blocks for new cellular components from substances associated with a redox status. In addition,one-carbon metabolism plays a critical role in the regulation of the redox state by producing glutathione. Therefore, antifolates and drugs that target one-carbon metabolism, have been used for treating cancers. C1 metabolism is critical for the maintenance of genomic stability through nucleotide metabolism as well as for the epigenetic control of DNA and histones, altered expression of which is a characteristic attribution of tumor cells.
Recently, C1 metabolic enzymes have been shown to be novel therapeutic targets for cancer. As shown in table 1, dihydrofolate reductase (DHFR), serine hydroxymethyltransferase (SHMT), and thymidylate synthase (TYMS) have been used as targets to develop antifolate antineoplastic agents. One-carbon units contribute to multiple downstream pathways that are known to or are likely to benefit cancer cell survival. Traditional antifolate chemotherapeutics have many deleterious side effects due to the importance of folate pathways in healthy proliferating cells and
Table 1: Established anti-folate antineoplastic agents (Newman A C and Maddocks O D K, 2017)
| Drug name | Targets | Therapeutic uses |
| Aminopterin | DHFR | Initially found to reduce leukaemic cells in children; no longer in use |
| Methotrexate | DHFR | Used to treat a wide range of neoplastic disease |
| Pemetrexed | DHFR, TYMS (and SHMT) | Non-small cell lung carcinoma; pleural mesothelioma |
| Pralatrexate | DHFR | Peripheral T-cell lymphoma |
| Raltitrexed | DHFR and TYMS | Metastatic colorectal cancer |
| 5-Fluoruoracil | TYMS | Used to treat a wide range of neoplastic disease |
One-carbon metabolism plays a significant role in
References:
- Kalhan S C. One-carbon metabolism, fetal growth and long-term consequences//Maternal
and Child Nutrition: The First 1,000 Days.Karger Publishers, 2013, 74: 127-138. - Ducker G S, Rabinowitz J D. One-
carbonmetabolism in health and disease. Cellmetabolism , 2017, 25(1): 27-42. - Konno M, Asai A, Kawamoto K, et al. Theone-carbon metabolism pathway highlights therapeutic targets
forgastrointestinal cancer. International journal of oncology, 2017, 50(4):1057-1063. - Newman
A C , Maddocks O D K. One-carbonmetabolism in cancer. British journal of cancer, 2017, 116(12): 1499.